Intra-suprasellar Rathke's cleft cyst: a case report and literature review
Dahmane Elhairech, Saloua Kanazy, Lmejatti Mohammed
Corresponding author: Dahmane Elhairech, Departments of Neurosurgery, Hassan II Medical Center, Agadir, Morocco 
Received: 15 Sep 2021 - Accepted: 27 Sep 2022 - Published: 29 Sep 2022
Domain: Neuro-oncology
Keywords: Rathke's cleft cyst (RCCs), Rathke's pouch, intra-suprasellar, surgery, case report
©Dahmane Elhairech et al. PAMJ Clinical Medicine (ISSN: 2707-2797). This is an Open Access article distributed under the terms of the Creative Commons Attribution International 4.0 License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cite this article: Dahmane Elhairech et al. Intra-suprasellar Rathke's cleft cyst: a case report and literature review. PAMJ Clinical Medicine. 2022;10:16. [doi: 10.11604/pamj-cm.2022.10.16.31631]
Available online at: https://www.clinical-medicine.panafrican-med-journal.com//content/article/10/16/full
Intra-suprasellar Rathke´s cleft cyst: a case report and literature review
&Corresponding author
Rathke´s cleft cysts are benign cystic lesions of the sellar and suprasellar region, forming out of remnant cells of the craniopharyngeal duct. Rarely diagnosed because they are often asymptomatic, sometimes they can be symptomatic by causing neuro-ophthalmological and/or endocrine disorders requiring surgical treatment. In this report we present a patient with a symptomatic Rathke´s cleft cysts in an intra-suprasellar location, and review of the literature.
Rathke's cleft cysts are benign lesions formed from residue of the embryonic Rathke pouch. Usually located in an intrasellar or intra-suprasellar region. The pathogenesis of these lesions remains a subject of debate. Most often asymptomatic with fortuitous discovery on routine magnetic resonance imaging. Rarely these lesions can be symptomatic and engender a mass effect on surrounding structures, causing headaches, endocrine dysfunction, or visual disturbances. Because of their variable radiological features at computed tomography (CT) and magnetic resonance imaging (MRI), preoperative diagnosis can be difficult. Once the cyst is symptomatic, surgery remains the treatment of choice by endonasal endoscopic surgery, or by the conventional microsurgical technique.
Patient information: a 23-year-old girl presented with complaints of visual impairment, and headaches for 8 weeks prior.
Clinical findings: detailed neurological examination was normal. The visual fields (with Goldmann perimetry) revealed a bitemporal hemianopsia. The patient´s hormonal status was normal.
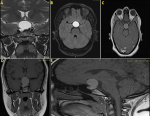
Diagnostic assessment: the preoperative MRI study of the sellar region, shown in Figure 1 (A,B,C,D,E), revealed a sellar and suprasellar lesion, hyperintense to brain on the T1-weighted image, with a slight enhancement of the outer border of the lesion, causing deformation of the adjacent optic chiasm and pressure effect on the third ventricule, which led to the provisional diagnosis of a cystic craniopharyngioma.
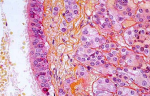
Therapeutic intervention: surgical removal of this lesion was felt to be indicated because a diagnosis could not be made otherwise in this young patient, and because compression of the optic chiasm was imminent causing her visual disturbance. A right pterional approach was chosen as the operative approach to this intra-suprasellar lesion. The Sylvian fissure was opened for exposure of the lesion, which was yellow, cystic, mostly avascular, and approximately 20 mm in diameter. Tissue fragments were fixed in 10% aqueous formalin. Examination revealed a single-cell layered, cuboidal epithelium and loose connective tissue. The final diagnosis was of a Rathke´s cleft cyst (Figure 2).
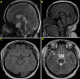
Follow-up and outcomes: the postoperative course was uneventful, the patient´s visual impairment and headaches are improved. The postoperative MRI study of the sellar region, shown in Figure 3(A,B,C,D), revealed gross-total resection of the tumor.
Rathke´s cleft cysts are well-delimited structures that vary in size from several millimetres to 1-2 cm [1]. Rathke´s cleft cyst (RCCs) are typically intrasellar, or intra and suprasellar, but may occur in an entirely suprasellar location with intact sella turcica. In the sella, Rathke pouch, formed during the fourth week of gestation, gives rise anteriorly to the adenohypophysis and posteriorly to intermediate lobe of the pituitary gland. In the suprasellar cistern, Rathke pouch gives rise to the pars tuberalis. If the normal involution of Rathke´s pouch fails to occur, the resulting RCC may increase in size by accumulation of cyst fluid and compress the surrounding structures, which can be symptomatic [2]. The median age of a patient diagnosed with symptomatic RCC is in the late 30s [3], with a female preponderance in the published series [4], but they have been reported in numerous pediatric patients [5], and as well as elderly patients in their 70s [3]. Asymptomatic RCCs are common incidental findings in 4-33% of autopsy cases and increasingly found on routine magnetic resonance imaging [6]. Rarely, these lesions can grow to become symptomatic, causing mass effect on surrounding structures, such as the pituitary gland, hypothalamus, and optic chiasm [7,8]. Comparisons of symptomatic and asymptomatic lesions have suggested that large size and suprasellar extension of RCCs may be associated with a greater tendency toward symptomatic presentation [1,3]. The typical symptoms include headache, endocrinologic dysfunction, or visual loss as our patient. In the published literature, headache was described in 44-81% of symptomatic cases [9], endocrinologic dysfunction in 30-60% of symptomatic cases such as hyperprolactinemia and growth hormone deficiency, or even diabetes insipidus, [10,11], and visual loss in 11-67% of symptomatic cases [12]. Intrasphenoidal RCC differs from intra-suprasellar RCC as it does not lead to visual loss or endocrinopathy [13]. Nearly all examples of RCCs with visual loss was due to a suprasellar extension [14].
The endocrinologic symptoms may differ depending on the age and gender of the patient. Men tend to suffer from hypogonadism, resulting in fatigue and decreased libido, while premenopausal women can present menstrual irregularities and galactorrhea, and postmenopausal women can present symptoms of panhypopituitarism, such as fatigue and altered mental status [15]. Diabetes insipidus is also a relatively common presenting finding in patients with RCCs, with reported rates ranging from 2.3% to 37% of patients [15]. Rathke´s cleft cysts are considered to be benign lesions. The smooth wall of an RCC is composed of a single-cell layer of cuboidal or columnar epithelium resting on a basement membrane [16]. Some RCCs exhibit inflammatory contents and squamous metaplasia, and this metaplasia is thought to be the reaction to chronic inflammation [17]. The fluid inside an RCC can vary, it can be clear, similar to cerebrospinal fluid with low protein content, in which case the cyst will rarely cause symptoms. Or it can be filled with mucinous fluid with high protein content, which is more frequently seen in symptomatic patients. During surgery, these lesions are frequently found to be yellow, waxy, solid masses [6]. The histological results are the same regardless of whether the RCC is intra- or suprasellar [16].
On MRI, RCCs will typically appear hyperintense on T2. However, their appearance on T1-weighted imaging can be either hyperintense, or hypointense [6]. Because of the variable signal intensities on MRI imaging, sometimes it´s difficult to distinguish RCCs from other saddle lesions. The main differential diagnosis of RCCs includes pituitary adenoma, pituitary cyst and cystic craniopharyngioma. The key feature distinguishing RCCs from an adenoma is the midline location of RCCs without stalk deviation, as well as the position of the cyst between the anterior and posterior glands when viewed from sagittal cuts [6]. It may not be possible to differentiate some RCCs from cystic craniopharyngiomas or pituitary adenomas with cystic components. Computer tomographic scans may sometimes reveal calcifications, suggesting the diagnosis of a craniopharyngiomas [18]. As a rule craniopharyngiomas (CPs), which are also thought to be derived from Rathke´s pouch occur in younger patients than RCCs [19]. However, for Shin et al. the age at initial surgery was almost the same for both, RCCs and Cps (37±14 years and 35±14 years, respectively) [20]. Rathke´s cleft cysts usually measure less than 10 mm in diameter, in another series, they ranged from 8 to 26 mm. While CPs tend to be larger when first detected, Radiologic examination of CPs typically reveals suprasellar location, calcifications, mural enhancement, and bony changes of the sellar confines. craniopharyngiomas unlike RCCs, tend to recur after partial excision and must, therefore, be removed in total.
The surgical indications of this condition include headaches, vision and visual field changes, enlarged cysts, pituitary endocrine diseases, and diagnosis uncertainty [7]. While indicating a need for surgical intervention, acute visual loss is considered an emergency. Partial resection of the wall and evacuation of cyst contents is the treatment of choice, in order to provide a symptomatic relief and to confirm the diagnosis. Further treatment such as postoperative radiotherapy or chemotherapy is not needed [17]. Some authors have supported the aggressive approach to remove the entire cyst wall despite the lower risk of recurrence and the higher rates of postoperative endocrine dysfunction. Endoscopic approach provides good lighting, with a large angle and close observation to distinguish the normal pituitary and the cystic wall. In addition, it results in reduced trauma, less complications, and quick postoperative recovery [7]. In intra-suprasellar RCCs, the endoscopic endonasal approach is considered the treatment of choice, while the transcranial approach has been indicated in cases of suprasellar location, or lack of resources [17]. Our surgical choice was based on the clinic, the young age, the surgeon's experience, the large volume of the cyst, and its important suprasellar extension.
Some authors have also described the technique of marsupialization, whereby after the decompression, the cyst wall is opened up widely and left open. Unfortunately, no study to date has compared the efficacy of marsupialization over simple decompression. The rates of permanent postoperative diabetes insipidus in series of cases treated with cyst drainage have been reported to range from 0 to 9% [4,9,15]. On the other hand, the rates of new-onset diabetes insipidus post aggressive approach are reported to be higher, ranging from 19% to as high as 69% [15]. The rates of cyst recurrence after surgical resection can vary, some studies report no recurrence [8], and some describe high rates, up to 42%.
Rathke´s cleft cysts are frequently small benign lesions, asymptomatic and fortuitous discovery. Sometimes they can cause neuro-ophthalmological and/or endocrine disorders. Once the cyst is symptomatic, surgery remains the treatment of choice. The indication of craniotomie is replaced by the progression of neuroendoscopic techniques. Even a partial removal seems to be sufficient and provides good symptomatic relief, since recurrences occur with a low rate.
The authors declare no competing interests.
Data collection: Dahmane Elhairech, Saloua Kanazy. Article revision and data analysis and interpretation: Dahmane Elhairech, Saloua Kanazy, Lmejatti Mohammed. All the authors read and approved the final version of the manuscript
Figure 1: (A) coronal T2-weighted image; (B) axial T2 flair-weighted image; (C) and axial; (D) coronal, (E) sagittal, T1-weighted images demonstrate a large sellar and suprasellar cystic mass with a slight peripheral enhancement
Figure 2: photomicrograph of surgical specimen; the cyst wall possesses a single-cell layered cuboidal epithelium
Figure 3: (A,B,C,D) postoperative MRI images showed gross-total resection of the tumor
- Shatri J, Ahmetgjekaj I. Rathke´s cleft cyst or pituitary apoplexy: a case report and literature review. Open Access Macedonian Journal of Medical Sciences. 2018;6(3):544. PubMed | Google Scholar
- El-Mahdy W, Powell M. Transsphenoidal management of 28 symptomatic Rathke´s cleft cysts, with special reference to visual and hormonal recovery. Neurosurgery. 1998 Jan;42(1):7-16; discussion 16-7. PubMed | Google Scholar
- Isono M, Kamida T, Kobayashi H, Shimomura T, Matsuyama J. Clinical features of symptomatic Rathke´s cleft cyst. Clin Neurol Neurosurg. 2001 Jul;103(2):96-100. PubMed | Google Scholar
- Aho CJ, Liu C, Zelman V, Couldwell WT, Weiss MH. Surgical outcomes in 118 patients with Rathke cleft cysts. J Neurosurg. 2005 Feb;102(2):189-93. PubMed | Google Scholar
- Jahangiri A, Molinaro AM, Tarapore PE, Blevins L Jr, Auguste KI, Gupta N et al. Rathke cleft cysts in pediatric patients: presentation, surgical management, and postoperative outcomes. Neurosurg Focus. 2011 Jul;31(1):E3. PubMed | Google Scholar
- Han SJ, Rolston JD, Jahangiri A, Aghi MK. Rathke´s cleft cysts: review of natural history and surgical outcomes. Journal of Neuro-Oncology. 2013:117(2):197-203. PubMed | Google Scholar
- Zhongzhong Jiang, Mengqiang Yu, Yugang Jiang, Yong Peng. Endoscopic endonasal resection of symptomatic Rathke cleft cysts: clinical outcomes and prognosis. Neurosurg Rev. 2019 Sep;42(3):699-704. PubMed | Google Scholar
- El-Mahdy W, Powell M. Transsphenoidal management of 28 symptomatic Rathke´s cleft cysts, with special reference to visual and hormonal recovery. Neurosurgery. 1998 Jan;42(1):7-16; discussion 16-7. PubMed | Google Scholar
- Lillehei KO, Widdel L, Arias Astete CA, Wierman ME, Kleinschmidt-Demasters BK, Kerr JM. Transsphenoidal resection of 82 Rathke cleft cysts: limited value of alcohol cauterization in reducing recurrence rates. J Neurosurg. 2011 Feb;114(2):310-7. PubMed | Google Scholar
- Kasperbauer JL, Orvidas LJ, Atkinson JL, Abboud CF. Rathke cleft cyst: diagnostic and therapeutic considerations. Laryngoscope. 2002 Oct;112(10):1836-9. PubMed | Google Scholar
- Mukherjee JJ, Islam N, Kaltsas G, Lowe DG, Charlesworth M, Afshar F et al. Clinical, radiological and pathological features of patients with Rathke´s cleft cysts: tumors that may recur. J Clin Endocrinol Metab. 1997 Jul;82(7):2357-62. PubMed | Google Scholar
- Kim JE, Kim JH, Kim OL, Paek SH, Kim DG, Chi JG et al. Surgical treatment of symptomatic Rathke cleft cysts: clinical features and results with special attention to recurrence. J Neurosurg. 2004 Jan;100(1):33-40. PubMed | Google Scholar
- Manish K Kasliwal, Sumeet G Duab, Aparna Harbhajankac, Sukriti Nagc, Miral D Jhaverib, Roham Moftakhar. Intrasphenoidal Rathke´s cleft cyst. Journal of Clinical Neuroscience. 2015 Oct;22(10):1678-82. PubMed | Google Scholar
- Potts MB, Jahangiri A, Lamborn KR, Blevins LS, Kunwar S, Aghi MK. Suprasellar Rathke cleft cysts: clinical presentation and treatment outcomes. Neurosurgery. 2011 Nov;69(5):1058-68; discussion 1068-7. PubMed | Google Scholar
- Benveniste RJ, King WA, Walsh J. Surgery for Rathke´s cleft cysts: technical considerations and outcomes. J Neurosurg. 2004 Oct;101(4):577-84. PubMed | Google Scholar
- Itoh J, Usui K. An entirely suprasellar symptomatic Rathke's cleft cyst: case report. Neurosurgery. 1992 Apr;30(4):581-4; discussion 584-5. PubMed | Google Scholar
- Matsushima T, Fukui M, Ohta M, Yamakawa Y, Takaki T, Okano H. Ciliated and goblet cells in craniopharyngioma. Light and electron microscopic studies at surgery and autopsy. Acta Neuropathol. 1980;50(3):199-205. PubMed | Google Scholar
- Sumida M, Uozumi T, Mukada K, Arita K, Kurisu K, Eguchi K. Rathke cleft cysts: correlation of enhanced MR and surgical findings. AJNR Am J Neuroradiol. 1994 Mar;15(3):525-32. PubMed | Google Scholar
- Naylor MF, Scheithauer BW, Forbes GS, Tomlinson FH, Young WF. Rathke cleft cyst: CT, MR, and pathology of 23 cases. J Comput Assist Tomogr. 1995 Nov-Dec;19(6):853-9. PubMed | Google Scholar
- Shin JL, Asa SL, Woodhouse LJ, Smyth HS, Ezzat S. Cystic lesions of the pituitary: clinicopathological features distinguishing craniopharyngioma, Rathke´s cleft cyst, and arachnoid cyst. J Clin Endocrinol Metab. 1999 Nov;84(11):3972-82. PubMed | Google Scholar