Contribution of the syndromic approach in the diagnosis of meningitis at the university hospital of Marrakech
Rabi Adil, Rokni Taoufik, Ihbibane Fatima, Rada Nourddine, Draiss Ghizlan, Bourouss Mounir, Tassi Nora, Bouskraoui Mohammed, Soraa Nabila
Corresponding author: Rabi Adil, Microbiology Department, University Hospital Center of Marrakech, Morocco 
Received: 13 Oct 2019 - Accepted: 05 Dec 2019 - Published: 14 Dec 2019
Domain: Bacteriology,Microbiology,Molecular Biology
Keywords: Meningitis, encephalitis, multiplex PCR
©Rabi Adil et al. PAMJ Clinical Medicine (ISSN: 2707-2797). This is an Open Access article distributed under the terms of the Creative Commons Attribution International 4.0 License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cite this article: Rabi Adil et al. Contribution of the syndromic approach in the diagnosis of meningitis at the university hospital of Marrakech. PAMJ Clinical Medicine. 2019;1:58. [doi: 10.11604/pamj-cm.2019.1.58.20642]
Available online at: https://www.clinical-medicine.panafrican-med-journal.com//content/article/1/58/full
Research 
Contribution of the syndromic approach in the diagnosis of meningitis at the university hospital of Marrakech
Contribution of the syndromic approach in the diagnosis of meningitis at the University Hospital of Marrakech
Rabi Adil1,&, Rokni Taoufik1, Ihbibane Fatima1, Rada Nourddine2, Draiss Ghizlan2, Bourouss Mounir3, Tassi Nora4, Bouskraoui Mohammed2, Soraa Nabila1,5
1Microbiology Department, University Hospital Center of Marrakech, Marrakech, Morocco, 2Pediatric Department A, University Hospital Center of Marrakech, Morocco, 3Pediatric Emergency Department, University Hospital Center of Marrakech, Marrakech, Morocco, 4Infectious Disease Services, University Hospital Center of Marrakech, Marrakech, Morocco, 5Faculty of Medicine And Pharmacy, University of Cadi Ayyad Marrakech, Marrakech, Morocco
&Corresponding author
Rabi Adil, Microbiology Department, University Hospital Center of Marrakech,
Marrakech, Morocco
Introduction: central nervous system (CNS) infections such as meningitis and encephalitis currently represent a real diagnostic and therapeutic challenge. The objective of this study is to evaluate the contribution of the syndromic approach in the rapid and targeted diagnosis of meningitis and meningoencephalitis at the University Hospital of Marrakech.
Methods: this is a prospective study conducted over a period of one year (February 2018 - February 2019), including all patients with lumbar puncture with a cytology greater than 5 elements/mm3 and patients admitted for suspicion of meningoencephalitis.
Results: during this period, 176 lumbar punctures were performed that met the criteria to multiplex PCR, 101 cases for suspicion of meningo-encephalitis and 75 cases for suspicion of meningitis. The etiology was confirmed in 23% of the LCS treated for suspicion of meningitis. Bacterial etiology dominated the pattern in 70.5% of cases followed by viral (23.5%) and fungal (6%) etiology. The etiology was confirmed in 14% of the LCS treated for suspicion of meningoencephalitis. Viral etiology dominated the pattern in 81% of cases followed by bacterial (12.5%) and fungal (6%) etiology.
Conclusion: their rapid and efficient diagnosis made it possible to set up an appropriate treatment by avoiding the unnecessary prescription of antibiotics.
Infections that involve the central nervous system (CNS), such as meningitis/encephalitis (ME), are severe clinical conditions associated with high rates of morbidity and mortality as well as significant long-term sequelae [1]. Meningitis/encephalitis (ME) may be caused by a wide variety of pathogens, including bacteria, viruses and fungi; clinical symptoms may vary (e.g. fever, headache to altered consciousness, neck stiffness and seizures) and often overlap with various infectious agents [1,2]. Early identification of ME causative pathogens has been proven to enable timely and appropriate treatment there by reducing death or permanent neurological damage (such as problems with vision and hearing, cognitive deficits, seizures and behavioral changes) [3,4]. Cerebrospinal fluid (CSF) analysis is crucial in the diagnosis of CNS infection. Currently, in order to identify a potential causative pathogen, microbiological diagnosis in combination with cellular and chemistry parameters in CSF (some findings may suggest the general category of the causative agent, e.g. bacterial versus viral or fungal) are evaluated [4]. In particular, traditional tests such as Gram stain (GS) with culture and pathogen-specific molecular method on CSF samples are used for the diagnosis of acute bacterial/fungal and viral CNS infections, respectively. However, this conventional approach is conditioned by a low diagnostic yield and slow turnaround time. The sensitivity of GS and culture is relatively low and could further be reduced in patients who have received empiric therapy [3]. In addition, in ME cases, an etiology is not always identified. This could also be due to the lack of targeted testing and the low volume of CSF samples [1,5]. To overcome these limitations, interest focused on the development of standardized molecular diagnostic tests for simultaneous detection of the most common agents of infectious ME requiring a small volume of CSF [6,7]. New strategies need to be implemented that help clinician with the initial therapeutic decisions, possibly sparing patient from unnecessary anti-infective treatment. One strategy is the multiplex PCR test which-with pre-manufactured kits-facilitates the rapid identification of a variety of infectious agents. Here we present our experience after 1 year of usage of novel multiplex PCR in the routine clinical setting of suspected central nervous system (CNS) infection. The aim of the study was to assess changed diagnostic and therapeutic procedures and evaluate the consequences of the introduction of Film Array® multiplex PCR. In addition, we wanted to identify the most effective way to use this new technique in the routine patient management.
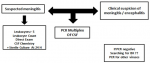
Study design and population: this prospective study was performed between February 2018 and February 2019, at Marrakech University Hospital Center. The suspected cases of acute meningitis were identified by a clinician, based on the following criteria: acute onset of fever (usually > 38.5°C rectal or 38.0°C axillary), headache and one of the following signs: neck stiffness, altered consciousness or other meningeal signs [7]. Newborns were enrolled in the study if the newborn has a fever accompanied by nonspecific symptoms (eg, poor feeding, vomiting, and diarrhea, rash) [8]. Multiplex PCR was performed for patients with a meningitis suspicion with cytology greater than 5 cells/mm3 and a sterile CSF culture after 24h. For patients admitted for suspicion of meningo-encephalitis, a PCR is performed if there is a strong clinical suspicion of encephalitis and if there are other biological abnormalities: procalcitonin, CRP, white blood cell count... (Figure 1).
Multiplex PCR: multiplex PCR on all samples was performed according to the manufacturer´s protocol (BioFire; FilmArray® Meningitis/Encephalitis (ME) PCR Panel) [9]. The CSF panel was used to detect 14 pathogens: CMV, enterovirus, HSV 1/2, HHV-6, human parechovirus, VZV, Cryptococcus neoformans/gattii, Escherichia coli K1, Haemophilus influenzae, Listeria monocytogenes, Neisseria meningitidis, Streptococcus agalactiae (GBS) and Streptococcus pneumoniae. The results were available approximately 75 min after the start of the assay in the bacterial laboratory. The transport of the samples from the clinical wards to the laboratory usually took about 10 min.
Statistical analysis: all statistical analysis was performed with SPSS version 11.5.
During the study period 1181 CSF was treated, the conventional method allowed the diagnosis of 71 bacterial meningitis with a positive CSF culture, and 1110 LCR had a sterile culture. A total of 176 lumbar punctures were performed that met the criteria to multiplex PCR, 101 cases for suspicion of meningo-encephalitis and 75 cases for suspicion of meningitis.
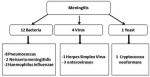
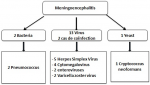
The demographic characteristics: 93 (53%) were from male patients and 83 (47%) were from female patients. Of all the 176 cases, 59 (34%) were of the age group < 15 years, 110 (62%) were 15-64 years, and 7 (4%) were > 65 years, 16% of patients were immunocompromised (HIV +).the demographic and laboratory characteristics of patients were included in this study are shown in Table 1. Of the 176 cerebrospinal fluids tested, 31 (18%) yielded a positive result by multiplex PCR, 17 cases for suspicion of meningitis and 14 cases for suspicion of meningo-encephalitis. The etiology was confirmed in 23% of the LCS treated for suspicion of meningitis. Bacterial etiology dominated the pattern in 70.5% of cases followed by viral (23.5%) and fungal (6%) etiology. Pneumococcus took first place with 47% of cases (Figure 2). The etiology was confirmed in 14% of the LCS treated for suspicion of meningoencephalitis. Viral etiology dominated the pattern in 81% of cases followed by bacterial (12.5%) and fungal (6%) etiology. Herpes simplex virus dominated the profile with 36% of cases (Figure 3). Positivity rate The FilmArray® ME Panel detected at least one pathogen in 31 of the 176 specimens that were tested, yielding an overall positivity rate of 18%, as shown in Table 2. The highest detection rates were in 15-34 age group and in the pediatric group (2-15 years). The most prevalent organisms detected during this study were S. pneumoniae (n = 10), Haemophilus influenza (n = 2), Neisseria meningitidis (n = 2), Enterovirus (n = 5), Cytomegalovirus (n=4), HSV 1 (n = 3), HSV 6 (n = 1), HSV 2 (n = 2), Varicella Zoster Virus (n = 2), and Cryptococcus neoformans (n = 2). Co-detections were observed in a two cases, the first case for VZV and HVS6 in a 1-month-old infant with varicella lesions who was contaminated by his 7-year-old brother who had chicken pox, and the second case for CMV and HVS6 in a positive HIV patient.
Meningitis and encephalitis often present with similar symptoms and the causative agents underlying the disease cannot be identified based on the clinical symptoms alone [10]. Diagnosis of meningitis and encephalitis has always been a challenge to laboratory mangers and clinicians. Currently available testing methods are time consuming and lack sensitivity and specificity. They are technically complex technologies and also require scientific expertise. Their accuracy may be affected by antibiotic administration prior to the testing, which is overcome by the FilmArray® technology. Also the small volumes of CSF obtained lead to a major challenge in the testing laboratory. Differentiation between bacterial, viral, or fungal meningitis/encephalitis is another challenge for clinical laboratories. While pleocytosis in the CSF is a sensitive marker of inflammation, several studies have shown that cell counts may be normal in both adult and pediatric patients despite an ensuing diagnosis of bacterial meningitis [11,12]. It is relevant to identify the etiologic agent in patients suffering from CNS infections. This would facilitate in identifying the most likely causative organisms. Diagnosis based on nucleic acid in vitro amplification-based molecular methods has broader and superior application in clinical microbiology practice. The Flmarray® ME panel is an US-FDA approved de novo technology, based on the principle of multiplex PCR with detection by melting curve analysis. It adequately detects significant number of targets and has high sensitivity and specificity compared to conventional techniques [13]. We analysed clinical and microbiological data from prospectively recruited patients with suspected meningitis in a teaching hospital in Marrakech. The study was performed within the routine clinical and laboratory settings of a hospital that had very limited prior experience with molecular techniques. In addition to the conventional laboratory investigations used in the hospital, a simple and rapid molecular diagnostic system was introduced to enhance laboratory diagnostics during the study period. To our Knowledge, this the first time a definite etiological diagnosis of viral meningitis has been made in patients in a public health facility in Marrakech. In this study, the overall positivity rate observed with the FilmArray® ME panel was similar to those described in other studies [13]. The etiology was confirmed in 14% of the LCS treated for suspicion of meningoencephalitis and in 23% of the LCS treated for suspicion of meningitis.
Virus were the most common etiological agents of CNS infections in this study with Enterovirus being the most common, followed by CMV and HSV-1 and VZV. Although the etiology is likely to vary between age groups and local epidemiology, this is in agreement with a study from Finland that found enterovirus, followed by HSV-2 and VZV to be major causes of aseptic meningitis in adults [14] and a study from Brazil that reported Enterovirus as the most common cause of meningitis followed by HSV-1, cytomegalovirus and dengue virus [15]. Early detection of etiologic agents improves the outcome of meningitis [16] and adequate laboratory diagnostics are imperative. Culturing of bacterial and fungal agents takes time and has low sensitivity, as illustrated by the fact that no one of 14 CSF samples with potentially cultivatable organisms was culture positive. The sensitivity of culture is affected by many factors including prior administration of antibiotics, suboptimal culturing conditions and media, and fastidious nature of some of the bacterial agents. Because of this and the unavailability of viral detection, virtually all patients with suspected meningitis in Morocco are treated as bacterial meningitis cases and the diagnosis is rarely reevaluated over the course of disease. This over diagnosis of bacterial meningitis inevitably leads to an overuse of antibiotics hence; rapid molecular diagnostics can have a major impact in low income settings by increasing the likelihood of reaching a correct diagnostics and enabling correct patient management. This is crucial not only for the outcome of the individual patient, but also for hospital biosecurity measures, public health decision and both local and global efforts to reduce and improve antimicrobial usage [17]. The introduction of syndromic testing of infectious diseases and fully automated multiplexed analyses represents a paradigm shift in microbiological diagnostics [18-20]. The FilmArray was able to detect microorganisms in 31 samples using the ME panel. Hence, such systems can improve patient management in settings with limited laboratory facilities. The FilmArray system was very easily implemented into a modestly equipped laboratory where personnel had little prior experience with molecular diagnostics.
However, there are a number of limitations to the sustained use of such automated systems in low-income countries. In Morocco, procurement of the necessary consumables is a complicated and lengthy process. The main obstacle, however, is the cost. Currently, the reagents needed of the analysis of one sample exceed 100 USD. Needless to say this is not sustainable in a public health system that is already financially constrained. On the other hand, a full course of treatment for suspected bacterial meningitis for 10-14 days [21-23], including only direct expenses for a hospital stay, routine investigations and antibiotic treatment, is likely to amount to more than 100 USD, even in Morocco. Another possible limitation of the system is the predefined selection of the pathogens in the panels. The panels were developed for an American ecology and may not be equally suited for Africa where other pathogens including M.tuberculosis are major cause of infections. It is also important that clinicians have a good understanding of test characteristics, interpretation of results and test limitations. Although lower than for conventional PCR, there is still a potential for sample contamination when using the FilmArray and the assay may detect latent or reactivated viruses [23-25]. The assays should be used with care and the positivity rates should be monitored. The FA ME panel test cannot supersede conventional microbiology procedures as it does not provide any information on antibiotic susceptibility. In addition, for virological analytes, quantitative results should be provided by the fllowing singleplex quantitative PCR to evaluate in the clinical context the relevance of virus detected [24-26]. The implementation of the microbiological diagnostic work up with sensitive and specific FA ME Panel testing may improve the management of patients with suspected CNS infection by early specific treatment and may be especially useful in cases that require effective prevention measures such as post-exposure prophylaxis of close contacts.
This study highlights the importance of this syndromic approach in the rapid confirmation of the etiologies of community meningitis and meningoencephalitis at the University Hospital of Marrakech. Their rapid and efficient diagnosis made it possible to set up and appropriate treatment by avoiding the unnecessary prescription of antibiotics. In addition, its reasoned use can make it possible, at no extra cost, to improve the management of patients.
What is known about this topic
- The Filmarray® ME Panel is an US-FDA approved de novo technology, based on the principle of multiplex PCR with detection by melting curve analysis. It adequately detects significant number of targets and has high sensitivity and specificity compared to conventional techniques.
What this study adds
- The aim of the study was to assess changed diagnostic and therapeutic procedures and evaluate the consequences of the introduction of Film Array® multiplex PCR. In addition, we wanted to identify the most effective way to use this new technique in the routine patient management.
The authors declare no competing interests.
All the authors have read and agreed to the final manuscript.
We thank all the team of the microbiology department for the good performance of the tests and the preservation of the files of the patients.
Table 1: characteristics of the study population and specimens analysed
Table 2:
positivity rate for the Film Array ME Panel for all samples and by age group
Figure 1: algorithm for
the management of CSF at the laboratory of the University Hospital Center of
Marrakech
Figure 2: isolated germs in meningitis
Figure 3: isolated germs in meningo-encephalitis
- Leber AL, Everhart K, Balada-Llasat JM, Cullison J, Daly J, Holt S et al. Multicenter evaluation of biofire filmarray meningitis/encephalitis panel for detection of bacteria, viruses, and yeast in cerebrospinal fluid specimens. J Clin Microbiol. 2016 Sep;54(9):2251-61. PubMed | Google Scholar
- Hanson KE. The first fully automated molecular diagnostic panel for meningitis and encephalitis: how well does it perform, and when should it be used. J Clin Microbiol. 2016 Sep;54(9):2222-2224. PubMed | Google Scholar
- Messacar K, Breazeale G, Robinson CC, Dominguez SR. Potential clinical impact of the Film Array meningitis encephalitis panel in children with suspected central nervous system infections. Diagn Microbiol Infect Dis. 2016 Sep; 86(1):118-120. PubMed | Google Scholar
- Bianchi L, Napoli Z, Donati S, Lencioni P, Santoni F, Riccardo Lari. Emergency management in bacterial meningitis and sepsis: application of real time-polimerase chain reaction and FilmArray technology performed directly on cerebrospinal fluid and blood samples. Microbiologia Medica. 2015;30(2):5084. Google Scholar
- Wootton SH, Aguilera E, Salazar L, Hemmert AC, Hasbun R. Enhancing pathogen identification in patients with meningitis and a negative Gram stain using the BioFire FilmArray® Meningitis/Encephalitis panel. Ann Clin Microbiol Antimicrob. 2016;15:26. PubMed | Google Scholar
- Piccirilli G, Chiereghin A, Gabrielli L, Giannella M, Squarzoni D, Turello G et al. Infectious meningitis/encephalitis: evaluation of a rapid and fully automated multiplex PCR in the microbiological diagnostic workup. New Microbiol. 2018 Apr;41(2):118-125. PubMed | Google Scholar
- WHO. Immunization surveillance. Assessment and Monitoring. 2013 Accessed October 19 2019.
- Nicole Le Saux and Canadian Paediatric Society, Infectious Diseases and Immunization Committee. Guidelines for the management of suspected and confirmed bacterial meningitis in Canadian children older than one month of age. Paediatr Child Health. 2014 Mar;19(3):141-146. Google Scholar
- Arora HS, Asmar BI, Salimnia H, Agarwal P, Chawla S, AbdelHaq N. Enhanced identification of group B Streptococcus and Escherichia Coli in young infants with meningitis using the biofire filmarray meningitis/encephalitis panel. Pediatr Infect Dis J. 2017Jul;36(7):685-687. PubMed | Google Scholar
- Thigpen MC, Whitney CG, Messonnier NE, Zell ER, Lynfield R, Hadler JL et al. Bacterial meningitis in the United States, 1998-2007. N Engl J Med. 2011 May 26;364(21):2016-25. PubMed | Google Scholar
- Fitch MT, van de Beek D. Emergency diagnosis and treatment of adult meningitis. Lancet Infect Dis. 2007 Mar;7(3):191-200. PubMed | Google Scholar
- Durand ML, Calderwood SB, Weber DJ, Miller SI, Southwick FS, Caviness VS Jr et al. Acute bacterial meningitis in adults: a review of 493 episodes. N Engl J Med. 1993 Jan 7;328(1):21-8. PubMed | Google Scholar
- Naccache SN, Lustestica M, Fahit M, Mestas J, Dien BJ. One year in the life of a rapid syndromic panel for meningitis/encephalitis: a pediatric tertiary care facility's experience. J Clin Microbiol. 2018 Apr 25;56(5). PubMed | Google Scholar
- Kupila L, Vuorinen T, Vainionpaa R, Hukkanen V, Marttila RJ, Kotilainen P. Etiology of aseptic meningitis and encephalitis in an adult population. Neurology. 2006 Jan 10;66(1):75-80. PubMed | Google Scholar
- Soares CN, Cabral-Castro MJ, Peralta JM, de Freitas MR, Zalis M, Puccioni Sohler M. Review of the etiologies of viral meningitis and encephalitis in a dengue endemic region. J Neurol Sci. 2011 Apr 15;303(1-2):75-9. PubMed | Google Scholar
- Bahr NC, Boulware DR. Methods of rapid diagnosis for the etiology of meningitis in adults. Biomark Med. 2014;8(9):1085-103. PubMed | Google Scholar
- Barnes GK, Gudina EK, Berhane M, Abdissa A, Tesfaw G, Abebe G et al. New molecular tools for meningitis diagnostics in Ethiopia-a necessary step towards improving antimicrobial prescription. BMC Infect Dis. 2018 Dec 20;18(1):684. PubMed | Google Scholar
- Abbott AN, Fang FC. Clinical impact of multiplex syndromic panels in the diagnosis of bloodstream, gastrointestinal, respiratory and central nervous system infections. Clin Microbiol Newsl. 2017;39:133-42. Google Scholar
- Helb D, Jones M, Story E, Boehme C, Wallace E, Ho K et al. Rapid detection of Mycobacterium tuberculosis and rifampin resistance by use of on-demand, near-patient technology. J Clin Microbiol. 2010 Jan;48(1):229-37. PubMed | Google Scholar
- Bansidhar T, Poonam D. FilmArray® meningitis/encephalitis (ME) panel, a rapid molecular platform for diagnosis of CNS infections in a tertiary care hospital in North India: one-and-half-year review. Neurol Sci. 2019 Jan;40(1):81-88. PubMed | Google Scholar
- Favaro M, Savini V, Favalli C. A Multi-Target Real-Time PCR Assay for Rapid Identi fication of Meningitis-Associated Microorganisms. MolBiotechnol. 2013 Jan;53(1):74-9. PubMed | Google Scholar
- Anna E, Alexandra H, Melanie M, Johannes H. Clinical benefits of introducing real-time multiplex PCR for cerebrospinal fluid as routine diagnostic at a tertiary care pediatric center. Infection. 2019 Feb;47(1):51-58. PubMed | Google Scholar
- Ramanan P, Bryson AL, Binnicker MJ, Pritt BS, Patel R. Syndromic panel-based testing in clinical microbiology. Clin Microbiol Rev. 2017 Nov 15;31(1). PubMed | Google Scholar
- Ferreira CES, Guerra JCC, Slhessarenko N, Scartezini M, Franca CN, Colombini MP et al. Point-of-care testing: general aspects. Clin Lab. 2018;64(1):1-9. PubMed | Google Scholar
- Dien Bard J, Alby K. Point-counterpoint: meningitis/encephalitis syndromic testing in the clinical laboratory. J Clin Microbiol. 2018;56(4):1-10. PubMed | Google Scholar
- Javali M, Acharya P, Mehta A, John AA, Mahale R, Srinivasa R. Use of multiplex PCR based molecular diagnostics in diagnosis of suspected CNS infections in tertiary care setting-a retrospective study. Clin Neurol Neurosurg. 2017;161:110-6. PubMed | Google Scholar