A successful conservative management of ruptured subcapsular hepatic hematoma with pre-eclampsia
Abderrahim Siati, Amine El alaoui, Abdelaziz Baidada, Aicha Kharbach
Corresponding author: Abderrahim Siati, Department of Gynecology and Obstetrics, Maternity Souissi Hospital, University Hospital Center Ibn Sina, University Mohammed V, Rabat, Morocco 
Received: 21 Oct 2019 - Accepted: 28 Oct 2019 - Published: 08 Nov 2019
Domain: Urgent Care Medicine,Obstetrics and gynecology,General surgery
Keywords: Liver hematoma, pre-eclampsia, packing, transfusion
©Abderrahim Siati et al. PAMJ Clinical Medicine (ISSN: 2707-2797). This is an Open Access article distributed under the terms of the Creative Commons Attribution International 4.0 License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cite this article: Abderrahim Siati et al. A successful conservative management of ruptured subcapsular hepatic hematoma with pre-eclampsia. PAMJ Clinical Medicine. 2019;1:8. [doi: 10.11604/pamj-cm.2019.1.8.20755]
Available online at: https://www.clinical-medicine.panafrican-med-journal.com//content/article/1/8/full
Case report 
A successful conservative management of ruptured subcapsular hepatic hematoma with pre-eclampsia
A successful conservative management of ruptured subcapsular hepatic hematoma with pre-eclampsia
Abderrahim Siati1,&, Amine El alaoui2, Abdelaziz Baidada1, Aicha Kharbach1
1Department of Gynecology and Obstetrics, Maternity Souissi Hospital, University Hospital Center Ibn Sina, University Mohammed V, Rabat, Morocco, 2Department of Maternal Intensive Care, Maternity Souissi Hospital, University Hospital Center Ibn Sina, University Mohammed V, Rabat, Morocco
&Corresponding author
Abderrahim Siati, Department of Gynecology and Obstetrics, Maternity Souissi
Hospital, University Hospital Center Ibn Sina, University Mohammed V, Rabat,
Morocco
Ruptured subcapsular hematoma of the liver is a rare complication of pregnancy, commonly associated with pre-eclampsia or HELLP syndrome (Hemolysis, Elevated Liver Enzymes, and Low Platelets Syndrome). We report a case of a 32-year-old woman, with no particular antecedents, admitted to our emergency department at 32 weeks of amenorrhea, for pre-eclampsia with epigastralgia. The liver ultrasound performed at admission was normal. In the presence of acute fetal distress, a caesarean section with midline vertical incision was performed urgently. The surgical exploration revealed massive haemoperitoneum with ruptured subcapsular hepatic hematoma. The management consisted of hepatic packing with massive transfusion. Removal of packing was performed on the fourth day after stabilization and regression of hepatic encephalopathy. Fortunately, the evolution was favorable and the patient was discharged after 4 weeks of hospital stay in stable condition. The ruptured subcapsular hematoma of the liver remains a very challenging situation and potentially life-threatening condition.
The subcapsular hematoma of the liver is a rare complication of pregnancy. Its incidence varies between 1/45 000 and 1/225 000 births [1,2]. Generally, this complication is associated with pre-eclampsia, HELLP syndrome or rarely acute hepatic steatosis [3,4]. The subcapsular hepatic hematoma leads to a significant maternal and fetal mortality. Its association with acute hepatic steatosis complicates the management and worsens the maternal prognosis. Indeed, the management should be multidisciplinary and conservative as much as possible.
A case of 32-year-old Moroccan woman, G3P3, no particular antecedents, admitted to our emergency department at 32 weeks gestation for headache, epigastralgia, nausea and vomiting. On clinical examination: she was conscious, apyretic, presented with jaundice and fatigue, high blood pressure (185/110 mmHg) associated with proteinuria by dipstick urinalysis, heart rate was 110 beats/min, a respiratory rate of 16 breaths/min. The symphysis-fundal height was 26 cm, with a presumed cephalic presentation. The cervix was closed without any contraction and no vaginal discharge. The sounds of the fetal heart were well perceived. Obstetrical ultrasonography showing a singleton live pregnancy with normal quantity of amniotic fluid. The gestational age was estimated at 32 weeks. The hepatic ultrasound was normal. Laboratory investigations showed elevated liver enzymes (AST: 1275 IU/L, ALT: 682 IU/L), conjugated bilirubin of 88 mg/l, platelet count of 55,000 cells/mm3, hemoglobin of 10.4 g/dl. The prothrombin rate was 68%, international normalized ratio (INR) of 1.5. There was an hypoglycaemia of 0.55 g / L. Hepatic serologies A, B, and C have been requested and will eventually be negative. According to the clinico-biological context, the diagnosis of acute hepatic steatosis was proposed. Firstly, the systolic hypertension was controlled by nicardipine and hypoglycemia was treated by glucose serum 10%. One hour after admission, the fetus had presented acute fetal distress with severe bradycardia. Under general anesthesia, a cesarean section was performed urgently. Through a midline vertical incision, a massive hemoperitoneum was revealed. After aspiration of 800 mL of hemoperitoneal fluid, a live female neonate was delivered weighing 1770g and the Apgar score was 5 and 8 in 1 and 5 min respectively. Then the baby was hospitalized in the neonatal resuscitation department and will be well.
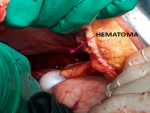
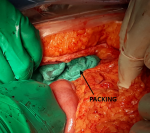
At the end of hysterorraphy, a maternal hypotension was observed relating to an intra-abdominal bleeding. The exploration showed an extensive ruptured subcapsular hematoma of the right liver lobe (Figure 1). In front of this hemorrhagic shock, the management consisted of vascular filling with saline serum 9‰, invasive monitoring of blood pressure, and administration of tranexamic acid (1g in 10 min) with oxytocin (40 IU in 30min). To stop bleeding, hepatic packing was performed (Figure 2). Also, uterus became flabby so prophylactic B-Lynch sutures were taken. Sub-hepatic drain on the right side and drain in pelvic space on the left side were performed. The patient received 6 units of red packed cells, 6 units of fresh frozen plasma and fibrinogen. These measurements allowed a stable hemodynamic state and a satisfactory coagulation. On the second postoperative day, the patient developed hepatic encephalopathy with worsening of renal insufficiency and pulmonary edema. The management of hepatic encephalopathy consisted of a control of intracranial hypertension with mannitol 10% and hypertonic saline serum 3%, diuretic therapy, correction of hyponatremia and hypoglycemia. Antibiotics were administrated for 10 days in front of bacterial pneumonia. In totality, the management consisted of damage control resuscitation procedure, with massive transfusion and conservative surgery. Additionally, the complications of massive transfusion have been prevented. The removal of packing was performed on the fourth day after stabilization and regression of hepatic encephalopathy. A liver biopsy was necessary to confirm acute hepatic steatosis, but infront of the risk of bleeding, this biopsy was abandoned. Evolution was marked by a regression of hepatic encephalopathy, an improvement of renal function and extubation at tenth day. The patient was discharged after 4 weeks of hospital stay in stable condition.
The subcapsular hematoma of the liver has an estimated incidence between 1/45 000 and 1/225 000 births [1,2]. Even though the mortality of this complication has decreased in recent years thanks to advances in resuscitation techniques and advances in the field of liver traumatology, it remains high for both the mother (1 to 24%) and for the child (6.7 to 70%) [5,6]. Most often it is a complication of pre-eclampsia, complicated or not with a HELLP syndrome. It occurs preferentially in the third trimester of pregnancy and postpartum (15 to 30% of cases) [7,8], in multiparous women, between 30 and 40 years on average. Signs of pre-eclampsia are usually unobtrusive or absent and may even be delayed in relation to hepatic symptomatology. The most consistent clinical sign (90% of cases) is persistent pain in the epigastrium or right hypochondrium more or less associated with scapular irradiation [9]. This pain is due to the distension of the hepatic parenchyma and Glisson's capsule following stasis of blood flow in the hepatic sinusoids. We often find a defense of the right hypochondrium. At the Glisson capsule rupture stage, signs of haemorrhagic shock (hypotension, tachycardia, oliguria) are associated with an acute surgical abdomen [9]. The presence of nausea and vomiting may be mistaken for a biliary or gastrointestinal pathology and thus a delay in management [10]. The biology is not specific, but it can reveal a complete or incomplete HELLP syndrome, coagulation abnormalities, up to a disseminated intravascular coagulation [11]. Abdominal ultrasonography and tomodensitometry (CT) are usually used for diagnosis [5]. In the emergency setting, ultrasound is readily available and can be used to directly identify the hematoma that most often begins in the right liver as a biconvex subcapsular lens [2]. The use of CT or magnetic resonance imaging, which is more efficient in liver exploration, is only possible in hemodynamically stable patients [7].The visualization of a haemoperitoneum in relation to a broken or fissured hematoma is then decisive in the therapeutic management [2]. Hepatic angiography, which can rarely be envisaged in emergency, is an alternative of choice for diagnostic and therapeutic purposes when clinical conditions allow it to be performed [12].
The therapeutic modalities of the capsular hematoma of the liver remain discussed, although they are more and more codified. All management must be fast and requires multidisciplinary collaboration. It includes three aspects, resuscitation associated with the treatment of hypertension, fetal extraction and treatment of subcapsular hematoma of the liver guided by imaging or abdominal exploration. Although surgery has long been considered the standard treatment for subcapsular hematoma of the liver, the range of its indications has decreased preferentially for conservative treatment. In our case, fetal distress revealed a haemorrhage of the liver and the hepatic ultrasonography was not efficient for the diagnosis and prepare the patient by correction of her coagulopathy and have an operative strategy (paking ready, consider embolization). In the context of ruptured subcapsular hematoma, caesarean section should be performed urgently for fetal extraction and placement of liver packing [3]. The latter allows 80% of maternal survival [1]. Then selective hepatic embolization is also effective with a maternal survival rate of 90% [1]. Other techniques such as surgical ligation of the hepatic arteries or resection of hepatic necrosis plaques are associated with significant maternal mortality above 30% [1]. Peri-operative resuscitation is based on the management of hemorrhagic shock by damage control procedure: vascular filling with crystalloid, invasive monitoring of blood pressure, monitoring of coagulation by routine tests or thrombo-elastometry if available to guide the treatment of coagulopathy. The blood transfusion must be given early by the red blood cells, the fresh frozen plasma and the platelets if they are less than 50000 / mm3 in a ratio of 1/1/1, fibrinogen and activated factor VII. Also, we should to correct metabolic acidosis, hypocalcemia associated with massive transfusion, hypothermia and use anti-fibrinolytics such as tranexamic acid [13]. In the postoperative management of encephalopathy, is based on mechanical ventilation, osmotherapy, sedation with benzodiazepines or propofol, a normalization of natremia and glycemia, a correction of coagulation. Diuretics to allow a preserved diuresis or prefering an extrarenal treatment with continuous hemodialysis. The prevention of gastrointestinal bleeding and antibiotic therapy for infections are necessary [14]. In the case of unruptured subcapsular hepatic hematoma, extraction of the baby is necessary after correction of coagulopathy, to stop the progression of hepatic lesions associated with pre-eclampsia. Postoperatively, the hematoma is strictly monitored to diagnose any fissures or rupture.
Ruptured subcapsular hepatic hematoma is a rare complication and representing a very challenging situation. Its gravity requires rapid diagnosis and appropriate multidisciplinary management. This clinical case illustrates the possibility of performing conservative surgical treatment.
The authors declare no competing interests.
All the authors have read and agreed to the final manuscript.
Figure 1: representing subcapsular hematoma of the right liver lobe
Figure 2: representing hepatic compression by packing
- Rinehart BK, Terrone DA, Magann EF, Martin RW, May WL, Martin Jr JN. Pre-eclampsia associated hepatic hemorrhage and rupture: mode of management related to maternal and perinatal outcome. Obstet Gynecol Surv. 1999; 54(3):196-202. PubMed | Google Scholar
- Wicke C, Pereira PL, Neeser E, Flesch I, Rodegerdts EA, Becker HD. Subcapsular liver hematoma in HELLP syndrome: evaluation of diagnostic and therapeutic options-a unicenter study. Am J Obstet Gynecol. 2004;190(1):106-1. PubMed | Google Scholar
- Sibai BM, Ramadan MK, Usta I, Salama M, Mercer BM, Friedman SA. Maternal morbidity and mortality in 442 pregnancies with hemolysis, elevated liver enzymes, and low platelets (HELLP syndrome). Am J Obstet Gynecol. 1993;169(4):1000-6. PubMed | Google Scholar
- Pereira SP, O'Donohue J, Wendon J, Williams R. Maternal and perinatal outcome in severe pregnancy-related liver desease. Hepatology. 1997;26(5):1258-1268. PubMed | Google Scholar
- Mihu D, Costin N, Mihu CM, Seicean A, Ciortea R. HELLP syndrome - a multisystemic disorder. J Gastrointestin Liver Dis. 2007 Dec;16(4):419-24. PubMed | Google Scholar
- Stevenson JT, Graham DJ. Hepatic hemorrhage and the HELLP syndrome: a surgeon's perspective. Am Surg. 1995 Sep;61(9):756-60. PubMed | Google Scholar
- Nunes JO, Turner MA, Fulcher AS. Abdominal imaging features of HELLP syndrome: a 10-year retrospective review. AJR Am J Roentgenol. 2005 Nov;185(5):1205-10. PubMed | Google Scholar
- Sibai BM, Ramadan MK, Usta I, Salama M, Mercer BM, Friedman SA. Maternal morbidity and mortality in 442 pregnancies with hemolysis, elevated liver enzymes, and low platelets (HELLP syndrome). Am J Obstet Gynecol. 1993 Oct;169(4):1000-6. PubMed | Google Scholar
- Pavlis T, Aloizos S, Aravosita P, Mystakelli C, Petrochilou D, Dimopoulos N et al. Diagnosis and surgical management of spontaneous hepatic rupture associated with HELLP syndrome. J Surg Educ. 2009 May-Jun;66(3):163-7. PubMed| Google Scholar
- Sibai BM, Taslimi MM, El-Nazer A, Amon E, Mabie BC, Ryan GM. Maternal-perinatal outcome associated with the syndrome of hemolysis, elevated liver enzymes, and low platelets in severe preeclampsia-eclampsia. Am J Obstet Gynecol. 1986 Sep;155(3):501-9. PubMed| Google Scholar
- Hunt CM, Sharara AI. Liver disease in pregnancy. Am Fam Physician. 1999 Feb 15;59(4):829-36. PubMed| Google Scholar
- Barton JR, Sibai BM. Gastrointestinal complications of pree-clampsia. Semin Perinatol. 2009 Jun;33(3):179-88. PubMed| Google Scholar
- Spahn DR, Bouillon B, Cerny V, Coats TJ, Duranteau J, Fernández-Mondéjar E et al. Management of bleeding and coagulopathy following major trauma: an updated European guideline. Crit Care. 2013 Apr 19;17(2): R76. PubMed | Google Scholar
- Stravitz RT, Kramer AH, Davern T, Shaikh AO, Caldwell SH, Mehta RL et al. Intensive care of patients with acute liver failure: recommendations of the U.S. Acute Liver Failure Study Group; Acute Liver Failure Study Group. Crit Care Med. 2007 Nov; 35(11): 2498-508. PubMed | Google Scholar