Cardiac tamponade: an unexpected onset of acute lymphoblastic leukemia (case report)
Zakaria Wakrim, Mohammed El Jamili, Oussama El Baroudi, Assala Cherki, Illias Tazi, Mustapha El Hattaoui
Corresponding author: Zakaria Wakrim, Department of Cardiology, Mohammed VI University Hospital, Faculty of Medicine, Marrakesh, Morocco 
Received: 05 Feb 2022 - Accepted: 05 Feb 2023 - Published: 07 Feb 2023
Domain: Cardiology,Oncology
Keywords: Cardiac tamponade, acute lymphoblastic leukemia, echocardiography, case report
©Zakaria Wakrim et al. PAMJ Clinical Medicine (ISSN: 2707-2797). This is an Open Access article distributed under the terms of the Creative Commons Attribution International 4.0 License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cite this article: Zakaria Wakrim et al. Cardiac tamponade: an unexpected onset of acute lymphoblastic leukemia (case report). PAMJ Clinical Medicine. 2023;11:33. [doi: 10.11604/pamj-cm.2023.11.33.31961]
Available online at: https://www.clinical-medicine.panafrican-med-journal.com//content/article/11/33/full
Cardiac tamponade: an unexpected onset of acute lymphoblastic leukemia (case report)
![]() Zakaria Wakrim1,2, Mohammed El Jamili2, Oussama El Baroudi1, Assala Cherki2,
Zakaria Wakrim1,2, Mohammed El Jamili2, Oussama El Baroudi1, Assala Cherki2, ![]() Illias Tazi1, Mustapha El Hattaoui2
Illias Tazi1, Mustapha El Hattaoui2
&Corresponding author
Prompt diagnosis of cardiac tamponade is of critical importance, as the underlying hemodynamic alteration may prove fatal. This case report describes the presentation of a young man with symptoms of heart failure, in whom cardiac tamponade was discovered as a harbinger of a hematologic malignancy. Further investigations confirmed the diagnosis of T-cell acute lymphoblastic leukemia. Despite chemotherapy, the patient developed severe complications and ultimately succumbed. The purpose of this report is to raise awareness that hematologic malignancies can manifest with pericardial effusions and to highlight the value of echocardiography in the evaluation of patients with leukemia. It is recommended that echocardiography be used not only for pre- and post-chemotherapy assessment, but also for routine detection of pericardial effusions and, in the event of hemodynamic compromise, to determine the timing, approach, and method of pericardial drainage.
Acute lymphoblastic leukemia (ALL) is a rare and aggressive form of blood malignancy that predominantly affects young adults. The development of this disease is believed to be the result of a complex interplay between exogenous or endogenous exposures, genetic predisposition, and chance [1]. The signs and symptoms of ALL are primarily the result of cytopenias caused by the accumulation of leukemia cells in the body. These symptoms often include fever, fatigue, bone and joint pain, and bleeding diathesis.
Pericardial effusion is a common complication of leukemia that can present with various clinical features. Typically, the presence of a malignancy is established before the identification of pericardial involvement. However, in some cases, a pericardial effusion may be the initial indication of the disease, highlighting the significance of early diagnosis for appropriate management [2]. Acute cardiac presentations of leukemia are an extremely rare occurrence, with only a limited number of cases reported in recent literature [3-7]. This report describes a case of ALL initially presenting with cardiac tamponade, a potentially life-threatening condition resulting from an excessive accumulation of fluid in the pericardial sac.
Patient information: a 23-year-old male patient hailing from a rural region was admitted to the cardiac intensive care unit due to symptoms of dyspnea, cough, and progressive chest pain over a period of 10 days, in addition to night sweats and a decline in overall condition. No history of flu-like symptoms, fever, or tuberculosis contagion was identified and no similar signs were observed among other members of the patient's family. It is worth noting that the patient had undergone pleural puncture for suspected (but not confirmed) pleural tuberculosis, as indicated by the presence of dyspnea, two weeks prior to his admission.
Clinical findings: on presentation, the patient exhibited acute orthopnea, with an oxygen saturation of 88% on room air, a blood pressure of 90/50mmHg, a regular heart rate of 150bpm, and a paradoxical pulse. Additionally, signs of right heart failure were observed, including spontaneous jugular vein distention, lower extremity edema, and significant ascites. Thoracic examination revealed dullness in the bases and faint heart sounds with no murmurs or rubs. A comprehensive evaluation also showed enlarged, non-tender axillary lymph nodes, discrete splenomegaly, and petechiae in both legs, with no cutaneous or gingival infiltrative syndrome.
Timeline: during the 1st week of March 2021: the patient underwent a pleural puncture to investigate the cause of dyspnea, which was suspected to be related to a case of tuberculosis. During the 3rd week of March 2021: the patient was admitted to the cardiac intensive care unit for further evaluation and management, where a diagnosis of tamponade was made. A pericardiocentesis revealed leukemic blasts in the fluid. During the 1st half of April 2021: the patient was transferred to the hematology department for complete follow-up, where T-acute lymphoblastic leukemia was diagnosed through bone marrow smears and immunophenotyping. During the 2nd half of April 2021: the patient was initiated on a cycle of cytoreductive chemotherapy, followed by a pediatric regimen of the GRAALL2013 protocol for treatment. However, the patient's condition rapidly worsened into a fatal outcome.
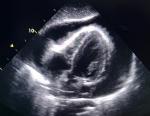
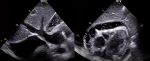
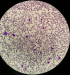
Diagnostic assessment and therapeutic interventions: a chest X-ray revealed marked cardiomegaly (CTI 0.7) with moderate bilateral pleural effusion. The electrocardiogram (ECG) demonstrated sinus tachycardia, diffuse micro voltage, and indications of electrical alternans. Given the patient's hemodynamic distress, an emergency transthoracic echocardiography (TTE) was performed and revealed a large pericardial effusion (Figure 1), with abnormal respiratory variation in transvalvular blood flow velocities, a paradoxical septum on inspiration, and a significant dilatation of the inferior vena cava, all consistent with the physiology of tamponade. In accordance with our triage strategy, an urgent pericardiocentesis was performed, resulting in the evacuation of 1000ml of hemorrhagic fluid. Chemical and cytological analysis of the fluid revealed an exudate with leukemic blasts (Figure 2). The patient's distress abated following the pericardiocentesis, as evidenced by satisfactory echocardiographic control (Figure 3). Further etiological testing, including investigations for infectious diseases such as tuberculosis and COVID-19, an inflammatory workup comprising a panel of antibodies and serological tests, a liver, renal, and thyroid assessment, and solid-tumor markers, were all found to be negative
The patient was then transferred to the hematology day hospital for further follow-up. The blood count finds: WBC (20G/L), HGB (10,1g/dL), and PLT (23G/L); the blood smear performed reveals 6% NEUT, 4% LYMPH, and 90% of small to medium-sized lymphoblasts with high nucleocytoplasmic ratio, fine chromatin, and sometimes prominent nucleoli, all in favor of acute leukemia; Bone marrow smears (Figure 4) and Immunophenotyping revealed an aspect of T-acute lymphoblastic leukemia. The hemostasis workup was normal, and the chromosomal formula on the hematological karyotype (according to ISCN 2016) was 47, XY, +3 del (9) (q22q32), -11, der (14) t (14) (q32), +mar (7)/46, XY (27); The FISH was pending. The patient was then started on prednisone 2mg/kg and allopurinol 400mg/d.
Follow-up and outcome of interventions: despite an overall improvement in the patient’s blood count following a cycle of cytoreductive chemotherapy (COP), symptoms persisted, including lymphadenopathy and pleural effusion. Consequently, the GRAALL2013 protocol was initiated prior to the availability of FISH results. On the fifth day of chemotherapy, the patient developed febrile neutropenia with respiratory distress and diffuse petechial purpura that progressed rapidly, ultimately leading to a fatal outcome despite supportive treatment. Workup data collected during the conditioning process indicated the presence of severe infectious pneumonitis in the context of acute renal failure.
Informed consent: the patient´s mother gave her informed consent and permitted the writing of this case report.
Pericardial effusions (PE) are a significant clinical entity, particularly in the context of cardiac tamponade, which can be life-threatening. The incidence of PE in leukemia is on the rise, with various etiologies such as hemorrhage, infection, leukemic infiltration, or chemotherapy having been reported [8]. Historically, PE has been considered a relatively infrequent presenting symptom in the diagnosis of leukemia [8]. However, it has been reported that roughly 20% of large symptomatic effusions without a definite etiology at baseline may represent the initial presentation of undiagnosed cancer [9]. Furthermore, among other commonly encountered causes of PE, neoplasias are more often the result of metastasis rather than primary cardiac tumors [10]. Cardiac tamponade results from a build-up of pericardial fluid under pressure, it comprises a continuum from simple effusion to circulatory collapse depending on the speed of fluid accumulation and compliance of the pericardium.
Typical signs of cardiac tamponade are embedded in Beck's triad: hypotension, jugular venous distension, and muffled heart sound. Additional symptoms of right heart failure may also be present. Electrocardiography (ECG) may reveal low voltage and electrical alternans, which are typical but not exclusive abnormalities. Chest radiography may be normal, or show an enlarged cardiac silhouette. Bedside doppler echocardiography has been shown to have high diagnostic accuracy in symptomatic patients and is considered the standard non-invasive, cost-effective method for the diagnosis of cardiac tamponade. Echocardiography can be used without delay to detect the effusion, assess its hemodynamic significance, visualize ventricular and atrial compression during the cardiac cycle, exclude differential diagnoses, and guide emergency pericardiocentesis and drainage [10]. In accordance with expert consensus statements [11], TTE is considered the backbone of cardiac evaluation in patients undergoing cancer therapy and should be used not only in the event of complications, but also in preparation for and during therapy.
Draining more than 1L of fluid from the pericardial space at once can be harmful, thus prolonged pericardial drainage through a catheter left in place overnight may be considered in some cases to prevent the recurrence of tamponade [10,12]. The technique is relatively safe, with limited contraindications and complications, especially when assisted by echocardiography. However, it is important to note that the survival rate for patients with malignant effusions with impending or recurrent tamponade is poor [2]. 75% of pericardial effusion patients also have pleural effusion, as shown by thoracic imaging studies on leukemia patients [8]. Our patient's delayed management of PE was due to the lack of cardiac evaluation following the pleural puncture.
Despite the unusual presentation, our patient's diagnosis of acute lymphoblastic leukemia was swiftly established, after ruling out other potential differential diagnoses, particularly tuberculosis. The T-cell type of ALL is a malignant disorder characterized by the massive clonal proliferation of immature lymphoid progenitor cells, as per the 2016 WHO classification of lymphoblastic leukemia/lymphoma [13]. The diagnosis is primarily based on the demonstration of surface/cytoplasmic CD3, as most T-cell markers are not entirely lineage-specific. Cytogenetics and analysis of cellular DNA content are utilized to identify submicroscopic leukemia type-specific abnormalities and guide therapy [1]. All structural abnormalities observed in our patient´s karyotype (trisomy 3 and del 9q) are reported in type T ALL. The chromosome 14 derivative could correspond to chromosome 11 (to be confirmed by FISH).
Once the diagnosis is established, individualized treatment should begin immediately. In general, pre-phase therapy, including corticosteroids alone or in combination with vincristine and cyclophosphamide (COP), is often given in conjunction with allopurinol and hydration for 5 to 7 days to reduce the tumor and prevent tumor lysis syndrome (TLS). Supportive therapy should be initiated as soon as necessary to treat infections or replace blood cells [14]. The next phase is the induction of complete remission (CR), with the goal of eradicating ALL cells with minimal toxicity [15]. In this case, the GRAALL protocol was chosen. Studies have shown that adolescents and adults may have better outcomes with pediatric regimens compared to adult ones [1].
The pathological process of ALL leads to bone marrow insufficiency by infiltration, resulting in severe cytopenia. If left untreated, the disease can be fatal within weeks. Multidrug chemotherapy for acute leukemia can lead to further myelosuppression and potential complications like tumor lysis syndrome and febrile neutropenia, which can result in various metabolic difficulties, including severe renal failure and cardiac arrhythmias. Rapidly spreading infections also pose a risk and require prompt, broad-spectrum antibiotic treatment. The patient in question presented with an unusual and rapidly progressing disease, which was resistant to COP therapy and complicated by severe pneumopathy, leading to a poor prognosis.
This case report describes an exceptional and premature onset of acute lymphoblastic leukemia in a patient who initially presented with cardiac tamponade and lacked any previous evidence of malignancy. This serves as a significant reminder of the need to consider this diagnosis in patients displaying new-onset pericardial effusion. The utilization of bedside doppler echocardiography as a diagnostic method can aid in early identification of pericardial involvement in these patients. Additionally, the authors emphasize the significance of a comprehensive echocardiographic examination in patients diagnosed with leukemia and the advantages of a multidisciplinary approach that involves the collaboration of various specialists for prompt diagnosis, appropriate administration of chemotherapy, and effective supportive treatment. This is particularly crucial in the management of high-risk patients, whose condition may rapidly worsen and necessitate vigilant and comprehensive care.
The authors declare no competing interests.
Patient management: Zakaria Wakrim and Oussama El Baroudi. Data collection: Zakaria Wakrim and Mohammed El Jamili. Manuscript drafting and completion: Zakaria Wakrim and Assala Cherki. Manuscript revision and final approval: Illias Tazi, Mustapha El Hattaoui Zakaria Wakrim, Mohammed El Jamili, Oussama El Baroudi, Assala Cherki, Illias Tazi and Mustapha El Hattaoui. All the authors have read and agreed to the final manuscript.
The authors would like to express their gratitude to Dr. Fatima Zahra Lahlimi from the Department of Hematology and Bone Marrow Transplantation and Dr. Hiba Boumaazi from the Laboratory of Biology for their invaluable contributions in the follow-up of the patient. Their participation has been essential to this case report.
Figure 1: large pericardial effusion: transthoracic echocardiogram subcostal view showing a large circumferential pericardial effusion with early diastolic right ventricle free-wall inversion
Figure 2: blasts in the pericardial fluid: evacuated fluid smear showing leukemic blast cells with no Auer rods
Figure 3: echocardiographic control after pericardiocentesis: transthoracic echocardiogram subcostal view revealing the presence of a residual mild pericardial effusion, in addition to an associated pleural effusion. It is noteworthy that the inferior vena cava is still dilated
Figure 4: bone marrow aspirate smear: the marrow is invaded by lymphoblasts of various sizes, with regular nuclei and fine chromatin No myeloid or erythroid precursors are seen
- Inaba H, Greaves M, Mullighan CG. Acute lymphoblastic leukaemia. Lancet. 2013 Jun 1;381(9881):1943-55. PubMed | Google Scholar
- Burazor I, Imazio M, Markel G, Adler Y. Malignant pericardial effusion. Cardiology. 2013;124(4):224-32. PubMed | Google Scholar
- Leptidis J, Aloizos S, Chlorokostas P, Gourgiotis S. Fatal cardiac tamponade as the first manifestation of acute myeloid leukemia. Am J Emerg Med. 2014 Oct;32(10):1294.e1-2. PubMed | Google Scholar
- Prihadi E, Hariman C, Janssens L. Acute cardiac tamponade: presenting sign of acute leukaemia. Acta Cardiol. 2011 Apr;66(2):251-3. PubMed | Google Scholar
- El Sayed MJ, Gharzuddin W, Kharfan-Dahaja MA. T-cell Acute Lymphoblastic Leukemia Presenting as a Cardiac Tamponade. Austin J Emergency & Crit Care Med. 2014;1(2):4.
- Chaves FP, Quillen K, Xu D. Pericardial effusion: A rare presentation of adult T-cell leukemia/lymphoma. Am J Hematol. 2004 Dec;77(4):381-3. PubMed | Google Scholar
- Karabel M, Söker M, Kelekçi S, Karabel D, Yel S, Bilici M. Cardiac Tamponade may be the First Symptom of Leukemia. Pediatr Hematol Oncol. 2014 Mar;31(2):157-9. PubMed | Google Scholar
- Sampat K, Rossi A, Garcia-Gutierrez V, Cortes J, Pierce S, Kantarjian H et al. Characteristics of pericardial effusions in patients with leukemia. Cancer. 2010 May 15;116(10):2366-71. PubMed | Google Scholar
- Refaat MM, Katz WE. Neoplastic Pericardial Effusion. Clin Cardiol. 2011 Oct;34(10):593-8. PubMed | Google Scholar
- Adler Y, Charron P, Imazio M, Badano L, Barón-Esquivias G, Bogaert J et al. 2015 ESC Guidelines for the diagnosis and management of pericardial diseasesThe Task Force for the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology (ESC)Endorsed by: The European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2015;36(42):2921-2964. PubMed | Google Scholar
- Plana JC, Galderisi M, Barac A, Ewer MS, Ky B, Scherrer-Crosbie M et al. Expert Consensus for Multimodality Imaging Evaluation of Adult Patients during and after Cancer Therapy: A Report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Journal of the American Society of Echocardiography. 2014;27(9):911-939. PubMed | Google Scholar
- Vakamudi S, Ho N, Cremer PC. Pericardial Effusions: Causes, Diagnosis, and Management. Prog Cardiovasc Dis. 2017 Jan-Feb;59(4):380-388. PubMed | Google Scholar
- Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016 May 19;127(20):2391-405. PubMed | Google Scholar
- Hoelzer D, Bassan R, Dombret H, Fielding A, Ribera JM, Buske C. Acute lymphoblastic leukaemia in adult patients: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016 Sep;27(suppl 5):v69-v82. PubMed | Google Scholar
- Bassan R, Hoelzer D. Modern Therapy of Acute Lymphoblastic Leukemia. JCO. 2011;29(5):532-543. PubMed | Google Scholar