Transoral stapled pharyngogastroplasty following gastric pull-up in pharyngo-oesophageal strictures: a case series
Uvie Ufuoma Onakpoya, Oghenevware Joel Eyekpegha, Olugbenga Olalekan Ojo, Oluwaseun Rukeme Akanbi, Abayomi Emmanuel Oguns
Corresponding author: Oghenevware Joel Eyekpegha, Department of Surgery, Obafemi Awolowo University Teaching Hospital, Ile-Ife, Nigeria 
Received: 08 Jun 2023 - Accepted: 19 Aug 2023 - Published: 24 Aug 2023
Domain: General surgery, Head, Neck and Reconstructive Surgery, Thoracic surgery
Keywords: Case series, corrosive strictures, pharyngo-oesophageal, caustic
©Uvie Ufuoma Onakpoya et al. PAMJ Clinical Medicine (ISSN: 2707-2797). This is an Open Access article distributed under the terms of the Creative Commons Attribution International 4.0 License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cite this article: Uvie Ufuoma Onakpoya et al. Transoral stapled pharyngogastroplasty following gastric pull-up in pharyngo-oesophageal strictures: a case series. PAMJ Clinical Medicine. 2023;12:47. [doi: 10.11604/pamj-cm.2023.12.47.40706]
Available online at: https://www.clinical-medicine.panafrican-med-journal.com//content/article/12/47/full
Case series 
Transoral stapled pharyngogastroplasty following gastric pull-up in pharyngo-oesophageal strictures: a case series
Transoral stapled pharyngogastroplasty following gastric pull-up in pharyngo-oesophageal strictures: a case series
![]() Uvie Ufuoma Onakpoya1,
Uvie Ufuoma Onakpoya1, ![]() Oghenevware Joel Eyekpegha2,3,&,
Oghenevware Joel Eyekpegha2,3,&, ![]() Olugbenga Olalekan Ojo1,
Olugbenga Olalekan Ojo1, ![]() Oluwaseun Rukeme Akanbi4,
Oluwaseun Rukeme Akanbi4, ![]() Abayomi Emmanuel Oguns2
Abayomi Emmanuel Oguns2
&Corresponding author
Corrosive pharyngo-oesophageal stricture is now one of the rarer diseases of the oesophagus. In patients with non-dilatable or post-failed dilation corrosive pharynx-oesophageal strictures, the surgical options are varied as surgeons strive to preserve both swallowing and breathing mechanisms whilst reducing the risks of aspiration. In this case series, we describe our surgical approach to dealing with this disease entity and its outcomes. This is a case series of 6 patients who underwent transoral stapled pharyngogastroplasty for corrosive pharyngo-oesophageal strictures within the last 10 years. In this paper, we discuss our surgical technique of transhiatal oesophagectomy combined with a transoral stapled pharyngogastric anastomosis as well as their outcomes. All six patients underwent the surgical procedure. Four of the patients required an additional 1 or 2 sessions of dilation in their follow-up period. One patient suffered from persistent aspiration post operatively due to a diseased larynx and required a tracheostomy and jejunostomy feeding. There was no in-hospital mortality. Transoral stapled pharyngogastroplasty is an option in the management of this disease entity, and surgeons may add it to their armamentarium. A discussion with the patient about the potential need for dilation at some point in the future should be held.
Corrosive stricture of the oesophagus is still a health challenge in some countries, particularly strictures caused by caustic soda [1,2]. Surgical management is usually challenging, as many of these patients come to the hospital malnourished and may require oesophageal replacement or bypass. When the strictures involve the pharynx, this difficulty increases significantly as the upper sphincter is the narrowest part of the oesophagus and the swallowing mechanism of the pharynx and larynx may be destroyed, leading to problems with aspiration. Several surgical techniques have been described in managing these difficult strictures, with each ascribed to promote swallowing whilst preventing aspiration. The published results have been variable. In this paper, we describe in accordance with the STROBE guidance [3], the presentation, and outcomes including postoperative dysphagia and aspiration in a series of 6 patients who presented with non-dilatable corrosive pharyngo-oesophageal strictures and underwent the technique of transhiatal oesophagectomy, gastric pull-up and a transoral stapled pharyngogastroplasty (TSPG) whilst preserving the larynx.
This was a 10-year retrospective (2012-2021), single centre, consecutive case series of all patients who had corrosive pharyngo-oesophageal stricture and required oesophageal replacement being deemed either unsuitable for or had failed dilation at the Obafemi Awolowo University Teaching Hospital, Ile-Ife, Nigeria. The diagnosis was made following a history of dysphagia after ingestion of corrosives and a barium swallow which showed the location and extent of the lesion. Final diagnosis of the proximal extent of the lesion was made at surgery following surgical exposure. Relevant clinical data obtained from hospital records and outcomes of the procedure, especially post operative dysphagia or aspiration, are recorded and discussed. Follow-up was for at least 12 months and included in-person visits or telephone calls. The goal of follow-up was to ascertain either the presence of dysphagia or aspiration, asides any other complications. Data were obtained from hospital case files and a pro forma designed for follow-up. Dysphagia was graded in this study using the Mellow and Pinkas scoring system [4] as: grade 0: able to eat normal diet/no dysphagia. Grade 1: able to swallow some solid foods with occasional difficulty. Grade 2: able to swallow only semi-solid foods. Grade 3: able to swallow liquids only. Grade 4: absolute/total dysphagia. This case series has been reported in line with the STROBE guidelines [3].
Surgical technique
Anaesthesia and positioning: all patients consented to surgery, which was performed by the consultant or resident under consultant supervision. Anaesthesia is achieved with a single lumen tube and standard monitoring including invasive arterial line for continuous blood pressure monitoring, which is important when dissecting the mediastinal oesophagus as changes in blood pressure from left atrial compression can be quickly identified. The patient is positioned supine with arms adducted and neck extended and rotated to the right.
Abdominal phase-incision and stomach mobilization: routine skin preparation and draping is done. A midline supraumbilical abdominal incision is made and carried through to the peritoneum. The stomach is identified and detached from the greater and lesser omentum along with its vascular supply, sparing the right gastroepiploic artery on which the gastric conduit is pedicled. Both vagus nerves are divided.
Cervical phase-incision and mobilization of cervical oesophagus: a left oblique neck incision is made parallel to the medial border of the sternocleidomastoid muscle and deepened through to the deep cervical fascia. The sternocleidomastoid muscle and the carotid sheath are retracted laterally. The omohyoid and middle thyroid veins are divided. The cervical oesophagus up to its junction with the pharynx is fully mobilised by blunt dissection, taking care not to injure the left recurrent laryngeal nerve.
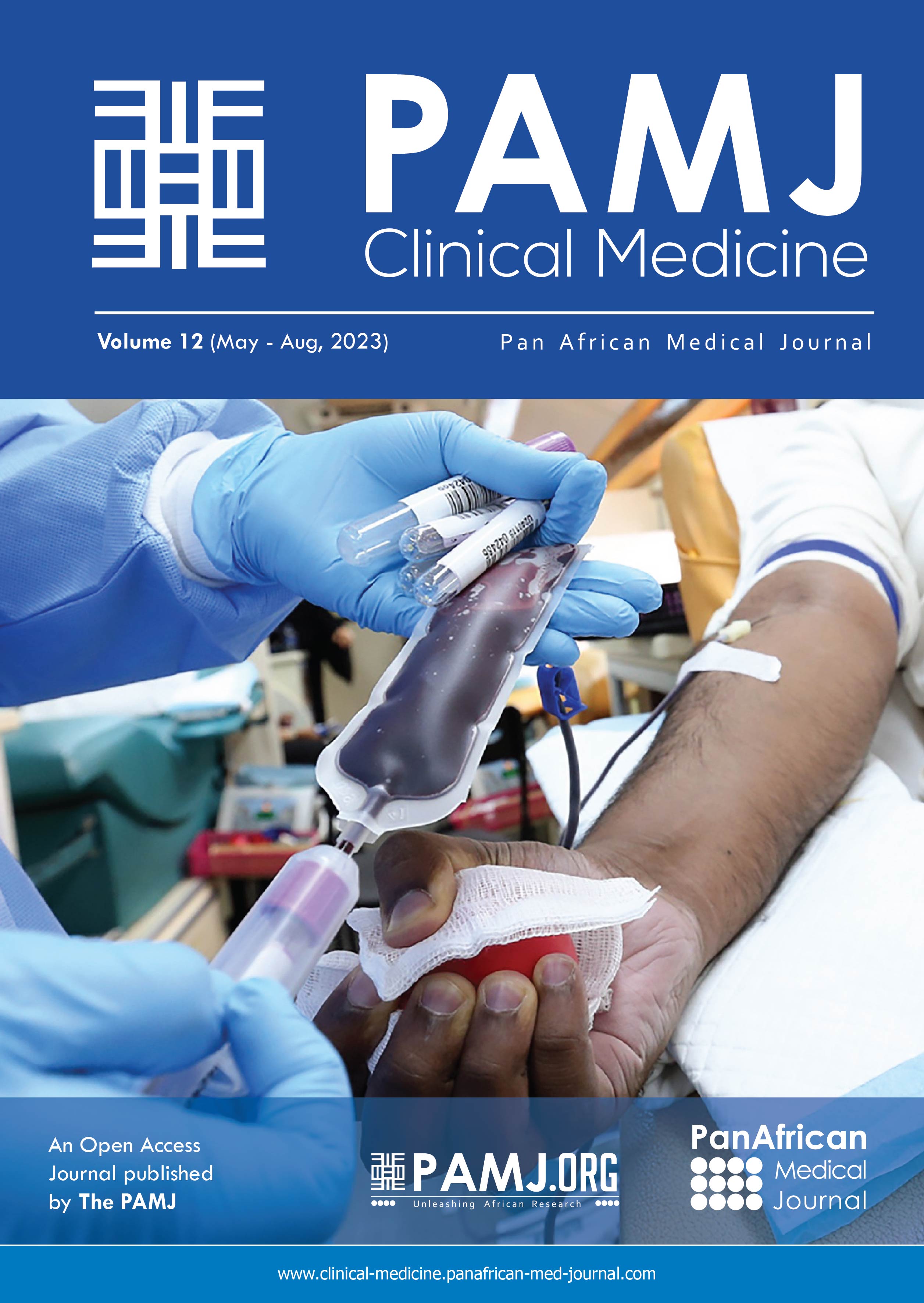
Mediastinal phase: the mediastinal oesophagus is mobilised by synchronous transhiatal and transcervical blunt finger dissection. The location of the stricture in the neck is identified by inspection, palpation and by an inability to advance a nasogastric tube beyond the point of stricture. Sometimes, a direct laryngoscopy/pharyngoscopy is required for identification of stricture location. The oesophagus is divided in the neck with a linear cutter and a tracker nasogastric tube sutured to its distal cut end which is then pulled into the abdomen still attached to the stomach (Figure 1) with the nasogastric tube maintaining the route in the posterior mediastinum.
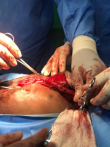
Conduit preparation and transport: the stomach is tubularised (Figure 2) creating a neo-oesophagus by excising portions of its lesser curvature and fundus along with the remnant oesophagus. A pyloromyotomy is performed. The tracker nasogastric tube is sutured to the stomach and pulled through the posterior mediastinum into the neck, bringing up the stomach to the neck.
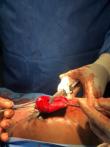
Construction of anastomosis: the normal part of the oesophagus is sized to estimate the appropriate stapler diameter. This was typically 21 mm in the children in this series and 25-27 mm in the adult patients. Two patients needed on-table dilation to achieve adequate sizing. An anterior gastrostomy is created near the apex of the neo-oesophagus, and the anvil of the stapler placed within it and secured with a purse string. The stapler is passed through the mouth by the surgeon and wedged against the strictured hypopharynx as distally as it can be advanced (Figure 3) with the assistant manipulating the stapler to ensure that it sits below the laryngeal inlet, thus reducing the risk and severity of aspiration. Once in place, the trocar of the stapler is deployed while directed posteriorly with the goal of piercing through the posterior wall of the strictured pharynx or oesophagus. The trocar is then coupled with the anvil and the stapler is fired to complete the anastomosis between the posterior wall of the hypopharynx and the anterior wall of the gastric conduit. The stapler is withdrawn and the tissue within it inspected to ensure a proper anastomosis has been performed. A nasogastric tube is advanced across the anastomosis to just below the diaphragm. A wound drain is placed in the neck and a feeding jejunostomy created. All the wounds are closed in layers.
Post-operative care: the patient feeds via the jejunostomy from the second postoperative day until oral intake is established. A barium swallow is performed on the 5th post-operative day to assess the integrity of the anastomosis. If there are no leaks, oral intake is commenced and established subsequently.
In the period reviewed, 6 out of 51 patients who had oesophageal replacement for corrosive oesophageal stricture were confirmed to have strictures involving the pharynx via pre-operative barium studies and intra-operative exposure of the oesophagus and pharynx in the neck. These 6 patients had the surgical procedure described above, and each case is summarised briefly.
Case 1: 23 years, female: she developed pharyngo-oesophageal strictures following accidental ingestion of caustic soda in childhood. She presented 21 years after the episode of caustic ingestion with grade 3 dysphagia and was referred to us following several failed dilations. A barium swallow was done, confirming a high stricture. Exposure of the neck at surgery confirmed a pharyngo-oesophageal stricture. She had transhiatal oesophagectomy and gastric pull-up with a TSPG constructed with a 25 mm circular stapler. She developed some dysphagia to solids 6 weeks after the primary surgery and was successfully dilated in one session. Follow-up 18 months later showed no further dysphagia and no aspiration.
Case 2: 2 years, female: she ingested an unidentified substance and presented with grade 3 dysphagia two months later. She had a feeding gastrostomy to optimise nutrition followed by transhiatal oesophagectomy, gastric pull up and TSPG with a 21 mm circular stapler after barium swallow and surgical exposure had revealed a pharyngo-oesophageal stricture with multiple distal oesophageal strictures. Now on follow up for 3 years after surgery, she reports no dysphagia or aspiration and takes normal diet.
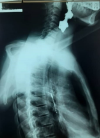
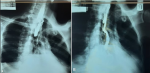
Case 3: 29 years, male: this patient presented with total dysphagia 3 weeks after accidental ingestion of caustic soda with a barium swallow confirming a post cricoid stricture (Figure 4) and following failed dilatation, a feeding gastrostomy was performed for nutritional optimisation followed by a TSPG 5 weeks after. Post-operatively (Figure 5), he remained dysphagia free for 5 months, following which he had some dysphagia to solids. He had 2 sessions of dilation and has remained dysphagia free after 6 years and 5 months of follow-up.
Case 4: 48 years, female: this patient ingested caustic soda 34 years prior and presented with grade 3 dysphagia. She opted for and had a transhiatal oesophagectomy, gastric pull up and TSPG with a 25 mm circular stapler. She developed an acute abdomen on the 5th post-operative day with an emergency laparotomy revealing a jejuno-jenunal intussuception with the lead point at the tip of the jejunostomy tube which was reduced. She commenced oral feeding after 10 days without problems. She developed grade 1 dysphagia, which responded well to a single session of dilation 6 weeks later. She also complained of aspiration, which improved following nutritional advice of early dinner and lying down with the head supported on pillows. She has been dysphagia free for 3 years and 3 months.
Case 5: 9 years, male: presented with grade 4 dysphagia 4 months after ingesting caustic soda granules accidentally. He required a preoperative feeding gastrostomy for nutritional rehabilitation after a failed attempt at oesophageal dilation. He subsequently had transhiatal oesophagectomy, gastric pull up and TSPG with a 21 mm circular stapler. Postoperative barium swallow done on the 5th day was satisfactory, following which he commenced oral intake. He does not complain of aspiration. Has now been followed up for 9 years since the surgery and has required 2 sessions of dilations, first at 2 months after surgery and 7 years after surgery following his growth into adulthood. His last session of dilation was 12 months ago, and he takes normal diet with no aspiration.
Case 6: 28 years, male: presented with grade 4 dysphagia and severe recurrent aspiration of feeds following suicidal ingestion of caustic soda 5 weeks prior. He had a barium swallow that showed a very high stricture with aspiration of contrast into the tracheobronchial tree bilaterally. He also had a preoperative bronchoscopy showing severely disfigured, fibrotic, scarred epiglottis, vocal cords, and hypopharynx. A transhiatal oesophagectomy, gastric pull up and TSPG was performed. He also required a tracheostomy with inflation of the cuff to treat troublesome aspiration and was subsequently discharged home with no oral intake and jejunostomy tube feeding only and for follow-up with the ENT surgeons. He, however, died unfortunately in uncertain circumstances whilst at home. There was no incidence of cervical anastomotic leakage or neck wound infection in any of the 6 patients who underwent surgery.
This case series describes our technique for dealing with a difficult disease. The surgical technique was successfully applied in all six patients, with 4 patients requiring a further 1-2 sessions of dilation for dysphagia, which successfully treated them. Only one patient suffered from troublesome aspiration, and this was attributed to the extensive laryngeal burns he suffered also. Corrosive oesophageal stricture is the commonest cause of oesophageal injury in Nigeria [5]. Corrosive injuries to the oesophagus mainly affect the mid-oesophagus with pharyngeal involvement seen less commonly [5,6]. Pharyngeal involvement of the disease markedly increases the complexity of the condition owing to the role the pharynx plays in swallowing, the likelihood of associated laryngeal injuries and scars and the overall significant risk of troublesome aspiration of meals. In addition, pharyngeal anastomosis is known to be prone to anastomotic stricture formation [7-9].
There are several factors to consider in determining the right method to apply in managing these patients. These include the availability of a residual lumen for dilation, the length of the stricture, the presence of multiple strictures distally and the degree of laryngeal scarification [7]. Replacement of all or a section of the oesophagus is usually reserved for patients with no residual oesophageal lumen, patients who have failed oesophageal dilatation and patients with multiple distal oesophageal strictures [7]. Several surgical techniques have been developed to deal with these kinds of pharyngeal strictures. They include serial dilation followed by oesophagocoloplasty or oesophagogastroplasty, myocutaneous flap pharyngoplasty, pharyngo-oesophagoplasty and primary colon flap augmentation pharyngo-oesophagoplasty (CFAP) [6,7,10]. All these techniques rely on extensive mobilization of the pharynx combined with a hand-sewn anastomosis performed in the neck. The goals of all these procedures are the same, namely, to resolve dysphagia and prevent aspiration. Given that pharyngo-oesophageal strictures are quite uncommon, and the patho-anatomy is variable, there is no single optimal surgical technique that is suitable for all patients. Restoration of gastrointestinal continuity with a pharyngogastric anastomosis has been described following pharyngolaryngectomy [11]. These patients however have an end tracheostomy stoma, eliminating the risk of aspiration after surgery. When the larynx is present as in these group of patients presented in the case series, then the risk of aspiration of food, either from a disfigured larynx, disturbance of the swallowing mechanism or anastomotic stenosis becomes a major concern [8].
One of the earliest descriptions of pharyngogastrostomy without laryngectomy for corrosive pharyngeal strictures is by Gupta [12]. Our technique is similar to what he described, with the exceptions being that we do not mobilise the entire laryngopharynx and our anastomosis is transoral stapled rather than transcervical handsewn. Performing a transoral anastomosis enables the surgeon to limit the extent of pharyngeal mobilisation. Most of the other methods of pharyngo-enteral anastomosis require a significant mobilisation and dissection of the pharynx. To achieve a satisfactory outcome with our technique, we seek to adhere to some general principles including: 1) adequate surgical exposure of the oesophagus and hypopharynx; 2) construction of a sufficiently wide anastomosis; 3) construction of a tension free cervical anastomosis preferably below the laryngeal inlet. The TSPG technique satisfies these conditions.
Results of other methods of managing these complex strictures are variable. Tettey in Accra [6] applied a hand sewn CFAP to 3 patients. Apart from an initial episode of problematic aspiration in 1 patient, all patients subsequently had good outcomes with no significant dysphagia or aspiration. Anantharishnan et al. [7] described a series of 51 patients with pharyngo-oesophageal stricture divided into several groups. Patients with dilatable strictures had dilation alone, whilst those who failed to achieve satisfactory swallowing had dilation followed by coloplasty. About 11 of the patients in his study with dense non-dilatable strictures had either a pectoralis major or sternocleidomastoid myocutaneous flap augmentation of the pharyngoesophageal stricture and appropriate treatment of more distal strictures. Six of these patients achieved normal swallowing, while another 2 had residual dysphagia to solids. In their review, unfortunately, 6 patients were deemed inoperable due to a lack of lumen beyond the pharyngo-oesophageal junction. As highlighted by Thomas and Dedo [9], the advantages of gastric replacement of the pharynx include a one stage procedure, single anastomosis in the neck without thoracic or abdominal anastomosis, excellent blood supply and healing, a large anastomotic stoma, resistance to negative intrapleural pressure and minimal peptic ulcer complications.
Restoration of gastrointestinal continuity in children with these condition poses 2 specific challenges. First, the diameter of the anastomosis is limited by the diameter of the pharynx and conduit and secondly, the anastomotic scar does not grow along with the child. One of the children who had TSPG in our study had his surgery when he was 8 years old. He is now 18 years and has required about 2 sessions of dilation within this period with relief of grade 1-2 dysphagia. Tannuri and Tannuri [13] in their series of 19 children who underwent hand-sewn pharyngo-coloplasty for pharyngo-oesophageal strictures reported that 9 of the children required further sessions of dilations due to anastomotic strictures with good relief of dysphagia.
Limitations: this was a case series and not a direct comparison with other methods of dealing with benign pharyngeal strictures and so inferences may not be drawn from this case series about the best method of treating this condition.
Transoral stapled pharyngogastroplasty (TSPG) is a viable technique for managing patients with pharyngeal corrosive strictures. It takes advantage of the fantastic properties of the stomach as a replacement conduit and advances in stapling technology to produce a durable and functional repair. Significant involvement of the larynx in the fibrotic process may be an indicator of troublesome aspiration, as has been shown from literature.
What is known about this topic
- Corrosive strictures involving both the pharynx and oesophagus are rare and much more difficult to treat than isolated oesophageal strictures. Most of the surgical procedures described to treat this condition are complex, prolonged, suture-based techniques requiring mobilization of the pharynx.
What this study adds
- This study describes a staple based technique deployed via the transoral route resulting in a shorter surgery time.
The authors declare no competing interests.
All authors contributed to the concept, design, manuscript writing and final approval of the article. All the authors have read and agreed to the final manuscript.
Figure 1: intraoperative photos: oesophagus has been mobilized into abdomen
Figure 2: intraoperative photos: tubularizing the stomach
Figure 3: wedging the circular stapler transorally across the pharyngeal stricture
Figure 4: barium swallow demonstrating a stricture at the cricopharyngeal region with prestenotic dilation and tertiary waves: surgical exposure confirmed stricture involving and extending from the upper oesophageal sphincter
Figure 5: postoperative barium swallow
- Ekpe EE, Ette V. Morbidity and mortality of caustic ingestion in rural children: experience in a new cardiothoracic surgery unit in Nigeria. ISRN Pediatr. 2012;2012:210632. PubMed | Google Scholar
- Fufore MB, Kirfi AM, Lawal J, Sani M. Clinical Spectrum and Implications of Corrosive Oesophageal Injuries in Northern Nigeria. Otolaryngol (Sunnyvale). 2020;10(388):2. Google Scholar
- Equator Network. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE)Statement: guidelines for reporting observational studies. 2023. Accessed June 15, 2023.
- Knyrim K, Wagner HJ, Bethge N, Keymling M, Vakil N. A controlled trial of an expansile metal stent for palliation of esophageal obstruction due to inoperable cancer. N Engl J Med. 1993;329(18):1302-7 PubMed | Google Scholar
- Thomas MO, Ogunleye EO, Somefun O. Chemical injuries of the oesophagus: aetiopathological issues in Nigeria. J Cardiothorac Surg. 2009 Oct 16;4:56. PubMed | Google Scholar
- Tettey M, Edwin F, Aniteye E, Tamatey M, Entsua-Mensah E, Offosu-Appiah E et al. Colon bypass with a colon-flap augmentation pharyngoesophagoplasty. Pan Afr Med J. 2015;21:275. PubMed | Google Scholar
- Ananthakrishnan N, Kate V, Parthasarathy G. Therapeutic Options for Management of Pharyngoesophageal Corrosive Strictures. J Gastrointest Surg. 2011;15(4):566-75. PubMed | Google Scholar
- Kurunkar S, Prabhu R, Kantharia C, Pujari S, Chaudhari V, Supe A. Corrosive pharyngoesophageal stricture - A challenge to surgeon: A tertiary center experience. Saudi Surg J. 2018;6:41-50. Google Scholar
- Thomas AN, Dedo HH. Pharyngogastrostomy for treatment of severe caustic stricture of the pharynx and esophagus. J Thorac Cardiovasc Surg. 1977;73(6):817-24. PubMed | Google Scholar
- Popovici Z. About Reconstruction of the Pharynx with Colon in Extensive Corrosive Strictures. Kurume Med J. 1989;36(1). Google Scholar
- Okamura A, Watanabe M, Kanamori J, Imamura Y, Takahashi K, Ushida Y et al. Digestive Reconstruction After Pharyngolaryngectomy with Total Esophagectomy. Ann Surg Oncol. 2021;28(2):695-701. PubMed | Google Scholar
- Gupta S. Total obliteration of esophagus and hypopharynx due to corrosives: a new technique of reconstruction. J Thorac Cardiovasc Surg. 1970 Aug 1;60(2):264-8. PubMed | Google Scholar
- Tannuri ACA, Tannuri U. Total esophageal substitution for combined hypopharyngeal and esophageal strictures after corrosive injury in children. J Pediatr Surg. 2017 Nov;52(11):1742-1746. PubMed | Google Scholar