Comorbidities associated with pediatric epilepsy at a Cameroonian tertiary teaching hospital: a cross-sectional study
Francklin Djifack Tetinou, Seraphin Nguefack, Félicitée Dongmo Nguefack, Nadia Adjifack Tetinou, Michael Ashu Agbor, Evelyn Mah, Andreas Chiabi
Corresponding author: Tetinou Djifack Francklin, Faculty of Medicine, Higher Institute of Health Sciences, Univeristé des Montagnes, Bangangté, Cameroon 
Received: 01 Mar 2020 - Accepted: 30 May 2020 - Published: 21 Jul 2020
Domain: Pediatric neurology
Keywords: Epilepsy, children, comorbidities, etiologies, Cameroon
©Francklin Djifack Tetinou et al. PAMJ Clinical Medicine (ISSN: 2707-2797). This is an Open Access article distributed under the terms of the Creative Commons Attribution International 4.0 License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cite this article: Francklin Djifack Tetinou et al. Comorbidities associated with pediatric epilepsy at a Cameroonian tertiary teaching hospital: a cross-sectional study. PAMJ Clinical Medicine. 2020;3:127. [doi: 10.11604/pamj-cm.2020.3.127.22094]
Available online at: https://www.clinical-medicine.panafrican-med-journal.com//content/article/3/127/full
Research 
Comorbidities associated with pediatric epilepsy at a Cameroonian tertiary teaching hospital: a cross-sectional study
Comorbidities associated with pediatric epilepsy at a Cameroonian tertiary teaching hospital: a cross-sectional study
Francklin Djifack Tetinou1,&, Seraphin Nguefack2, Félicitée Dongmo Nguefack2, Nadia Adjifack Tetinou1, Michael Ashu Agbor1, Evelyn Mah1, Andreas Chiabi1
&Corresponding author
Introduction: epilepsy is responsible for a significant proportion of disease burden globally. Comorbidities of epilepsy alters the quality of life of patients. There is paucity of literature in Cameroon on comorbidities in children with epilepsy. The aim of our study was to describe the comorbidities and etiologies of pediatric epilepsy at the Yaounde Gyneco-Obstetric and Pediatric Hospital.
Methods: this was a cross-sectional study using consecutive sampling techniques, carried out on 159 children aged 0-17 years old with epilepsy who were interviewed and examined at the Yaounde Gyneco-Obstetric and Pediatric Hospital, Cameroon. The data were collected from patient files and during routine outpatient visits examination. We calculated prevalence ratios of epilepsy, comorbidities, and their 95% confidence intervals and used log-binomial regression to calculate adjusted prevalence ratios.
Results: the prevalence of epilepsy was 5.9 %. The mean age was 6.1 years (SD 4.4). Hypoxic-ischemic encephalopathy 45(28.3%) was the leading cause of epilepsy. Mental retardation 83(52.2%), cerebral palsy 50(31.4%), attention-deficit/hyperactivity disorder 48(30.2%), and malnutrition 38(23.9%) were the main comorbidities observed in children with epilepsy. The prevalence ratios of mental retardation (OR =22.27, 95% CI 3.9-473.0, p= 0.000) and cerebral palsy (OR 18.20, 95% CI 5.29-80.31, p= 0.000) were greater in patients with infantile spasms. The others epileptic encephalopathies were significantly associated with buccofacial apraxia (OR 13.70, p=0.000), cerebral palsy (OR 13.38, p=0.000), and malnutrition (OR 3.59, p=0.04).
Conclusion: mental retardation, cerebral palsy, attention-deficit/hyperactivity, and malnutrition are the most common comorbidities among epileptic children in Cameroon.
More than 50 million people worldwide suffer from epilepsy with 80% of them living in low-and middle-income countries. The prevalence of epilepsy in Africa varies between 10 and 59 per 1,000. This number is twice that of high-income countries [1,2]. The higher prevalence of epilepsy in Africa is due to the high prevalence of central nervous system (CNS) infections, perinatal asphyxia and subsequent hypoxic-ischemic encephalopathy (HIE) [3]. Epilepsy adversely affects the clinical, social and economic wellbeing of both patients with epilepsy and their families. In sub-Saharan Africa, children with epilepsy are often stigmatized and the cost of their treatment is often borne by their families. Disorders that co-occur frequently with a principal disease are called comorbidities. Some comorbidities aggravate the clinical, social and economic wellbeing of children with epilepsy especially in those with epileptic encephalopathy. Between 26.8% and 84% of patients with epilepsy have a comorbid condition [4]. Mental retardation, psychiatric disorders, and malnutrition are more frequent in children with epilepsy than in those not having it [5-7]. Early recognition and management of the comorbidities of epilepsy can lead to a better quality of life and better outcomes [8]. In Cameroon, the prevalence of epilepsy is estimated at 5.8% and it constitutes 1.8% of all pediatric consultations [3,9]. To our knowledge, no study has been carried out on comorbidities in Cameroonian children with epilepsy. To this effect, we decided to assess the main etiologies and comorbidities of pediatric epilepsy at the Yaounde Gyneco-Obstetric and Pediatric Hospital (YGOPH), in Cameroon.
Type of study: this was a descriptive cross-sectional study carried out at the Yaounde Gyneco-Obstetric and Pediatric Hospital, Cameroon between march to july , 2019. The YGOPH is a tertiary level facility in the capital of Cameroon. Our study reviewed data on all outpatient children aged 0 to 17 years diagnosed with epilepsy by neuropediatrician and followed-up at the YGOPH. Epilepsy was defined as “two episodes of unprovoked seizures at least 24 hours apart”. Patients who did not undergo a full physical examination, who were hospitalized or who refused to participate in the study were excluded from the study. The Cochran formula was used to calculate the sample size assuming an expected proportion of subjects with epilepsy of 5.8/1,00 [9] and a p-value at 0.05. Using these values, we calculated the sample size to be 84. We used a pre-designed sheet to collect sociodemographic, clinical and therapeutic patient data from outpatient registers and patient files. Complementary patient data were collected in the clinic by a neuropediatrician. Each patient underwent detailed clinical examinations to identify epileptic syndromes, comorbidities and possible etiologies of the epilepsy. Weight was measured using electronic scales, height was recorded using height measuring scales and mid-upper arm circumference was measured using a flexible non-stretchable measuring tape. The Denver II tool was used to assess mental development and was supplemented by the Binet-Simon test for children over 6 years of age. At the end of the evaluation, we determined the developmental age (DA) and any mental retardation was diagnosed using the ICD-10 classification [10]. The authors assessed depression using the Patient Health Questionnaire-9 [11]. Epileptic encephalopathy was defined as a severe form of epilepsy characterized by severe seizures and profound neurological deficits.
Data analysis: the study data were collected and analyzed using Epi Info 3.5.4 (CDC, Atlanta) and WHO Anthro Survey (WHO, Geneva), with which we generated growth-weight curves. The Chi-squared and Fisher´s Exact tests were used to compare the association between epileptic syndromes and comorbidities, and between etiologies of the epilepsy and comorbidities. The prevalence ratios (odds ratios) and their 95% confidence interval were used to assess the strength of the association between the variables. Log-binomial logistic regression was used to calculate adjusted prevalence ratios.
Ethical considerations: the institutional review boards of the Higher Institute of Health Sciences in Bangangte, Cameroon (Ref No. 2019/044/UdM/PR/CIE) and YGOPH (Ref No 818/CIERSH/DM/2019), granted ethical clearances before starting the study. Patients and their parents were informed of the purpose and risks of the study and their approval was appropriately sought.
Our study population was made up of 159 individuals; 83(52.2%) patients were male, the mean age was 6.1 (SD 4.4) and ranged from 0-17 years (Table 1). The prevalence of epilepsy was 5.9 %. The most common etiology of the epilepsy was hypoxo-ischemic encephalopathy (28.3%, n=159). Infantile spasms (12.6%), frontal epilepsy (11.3%), temporal epilepsy (9.4%) and other epileptics encephalopathies (7.5%) were the most frequent epilepsy syndromes. Comorbidities associated with pediatric epilepsy were mental retardation (52.2%), cerebral palsy (31.4%), attention deficit hyperactivity disorder (30.2%), malnutrition (23.9%), buccofacial apraxia (23.3%) and depressive syndrome (9.4%). Table 1 further details the sociodemographic characteristics and associated comorbidities (Table 1), Figure 1,Figure 2, Figure 3, Figure 4, Figure 5, Figure 6, Figure 7. Infantile spasms was associated with mental retardation (OR 22.3, 95% CI 3.9-473.0, p=0.000) and cerebral palsy (OR 18.2, 95% CI 5.3-80.3, p=0.000). There was an association between the others epileptic encephalopathies and cerebral palsy (OR 13.4, 95% CI 3.0-91.5, p=0.000), buccofacial apraxia (OR 13.7, 95% CI 3.5-51.5, p=0.000), and malnutrition (OR 3.6, 95% CI 1.0-12.4, p=0.04) (Table 2). HIE was significantly associated with the following comorbidities: mental retardation (OR 4.8, 95% CI 2.2-11.0,p=0.000), buccofacial apraxia (OR 3.8, 95% CI 1.3-11.0, p=0.01), cerebral palsy (OR 11.1, 95% CI 4.9-24.9,p=0.000), and malnutrition (OR 2.31, 95% CI 1.1-5.0, p=0.03) (Table 2). Log-binomial regression analysis was used to control for the confounders of the factors that showed a significant association. Following this calculation, the adjusted prevalence ratio of HIE in cerebral palsy remained significant (p=0.000) (Table 3).
Sociodemographic characteristics: we found a hospital prevalence of 5.9% for pediatric epilepsy. This is superior to that reported by Mbonda et al. [3] in pediatric consultations at the Yaoundé Central Hospital (Cameroon) which was 1.8%. It is also higher than the prevalence reported by Traoré et al. [12] in Mali in children aged 3 to 15, which was 1.1%. Our high prevalence can be due to the fact that, YGOPH has a neuropediatric department with an important neuropediatric activity. Comparably to our findings, studies in the Republic of Benin, Uganda and Cameroon found that males were more affected by epilepsy than females [7,13]. The predominance of males has been attributed to genetic, hormonal and neurobiological differences in the neurons of male subjects which make them to be more sensitive to hypoxemia and then predispose them to develop hypoxic ischemic encephalopathy, which is the leading cause of epilepsy in our study [14-16]. Experimental studies of animals and patients suffering from stroke suggest that the sex hormones, in particular, the estrogens provide protection against hypoxic-ischemic cerebral lesions
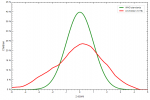
Height and weight curves: the weight-for-age, and height-for-age curves of children with epilepsy were left shifted to the WHO reference. The median of the height-for-age and weight-for-age curves of children with epileptic encephalopathies were left-shifted in comparison to the curves of children without epileptic encephalopathy. This shows that children with epilepsy are malnourished than children without epilepsy and the malnutrition is more pronounce amongst those with epileptic encephalopathy. This malnutrition can be explained by the high prevalence of buccofacial apraxia and the increase in the resting energy expenditure due to cerebral palsy and seizures in those children [17].
Etiologies of epilepsies: hypoxic-ischemic encephalopathy is the leading cause of epilepsy in our setting (28%). The high proportion of HIE is in part due to the lack of universal access to safe deliveries. As many as 79% of Cameroonian mothers from the poorest quintile of the population lack access to skilled birth attendants and 49% of births in urban areas are unsupervised by trained personnel [18]. In addition, many hospitals lack the equipment to adequately manage neonatal emergencies. They are often obliged to transfer patients to tertiary facilities, and this further delays management and increases the severity of the brain lesions.Even though infectious causes (cerebral malaria, CMV, meningitis and neonatal infection) accounted for 18.2% of childhood epilepsies, there were no cases of epilepsy due to HIV, tuberculosis and toxoplasmosis. Tuberculosis and toxoplasmosis are strongly associated with HIV. The absence of HIV, tuberculosis and toxoplasmosis as causes of childhood epilepsy reflects the success of the strategies implemented to curb the progression of HIV among pregnant women and infants. More than three quarters (80%) of pregnant women living with HIV in Cameroon have access to antiretroviral therapy and 61% of infants born with HIV are tested before their second month of life [19].
Comorbidities associated with epilepsy: half (52.2%) of the children in our study had mental retardation and every child with epileptic encephalopathy had mental retardation. Epileptic encephalopathies are severe forms of epilepsy which are accompanied by significant neurological deterioration [20]. As many as 98% of patients with infantile spasms have mental retardation [21]. Cerebral palsy represented the second comorbidity in our study, affecting 31.4% of children. Other studies have reported the frequency of cerebral palsy in children with epilepsy to be between 15 and 30% [20]. The high proportion of cerebral palsy is due to the equally high proportion of perinatal asphyxia as evidenced by the number of children with HIE in our study. Another common comorbidity was attention-deficit/hyperactivity disorder (ADHD). A third of our patients had ADHD, and this is similar to the findings of Rochelle et al. who found that 26% of pediatric epilepsies are associated with ADHD [22]. Williams et al. [5] ] observed higher values ranging between 20 and 50%. ADHD can be caused both by seizures and antiepileptic drugs particularly phenobarbital, benzodiazepines, phenytoin, carbamazepine, valproate, topiramate and zonisamide [23,24]. Almost every patient in our study took carbamazepine or valproate for their epilepsy. Phenobarbital and benzodiazepines are cheaper and more readily available. As a result, they are often prescribed by non-neuropediatricians as the first-line medications for epilepsy. It is, therefore, possible that ADHD is more frequent among children with epilepsy in Cameroon. Depression accounted for 9.4%, less than the 26% reported by Alan et al. [23]. This difference could be explained by the fact that the diagnosis of depression is particularly difficult before the age of 5 years. Alan studied children aged 7 to 18 while we worked on a younger population. 52% of children in our study were less than 5 years. Depression in children with epilepsy can be due to stigmatization and and lack of affection from parents who feel overwhelmed and may not know how to react with and around their children. Furthermore, some antiepileptic treatments can increase the risk of developing depression among patients with epilepsy [25,26].
We equally found that 3.8% of children had an autism spectrum disorder. This figure is much lower than than the findings of Williams et al. (21%) [2]. This difference can be due to the large proportion of children with severe mental retardation in our population (47%) in whom the clinical signs of autism spectrum disorders are difficult to objectify. Malnutrition was found in 23.9% of children with epilepsy. Malnutrition affects 22.1% of children with epilepsy in Benin [7] and 25.4% of children with epilepsy in France [27]. The high prevalence of buccofacial apraxia might explain the high proportion of malnutrition among children in our cohort given that it is characterized by impaired buccofacial movements which cause abnormal movements of the tongue and lips, which can lead to chewing and swallowing problems, making feeding difficult [28]. Another possible explanation for the high prevalence of malnutrition could be the lack of autonomy of children with epilepsy, particularly those suffering from cerebral palsy. Stiff limbs and spasticity increase the resting energy expenditure patients with cerebral palsy, further increasing the demand for energy and the chances of developing malnutrition [17].
Comparative analysis: infantile spasms multiplied the risk of mental retardation by 22 and was significantly associated with cerebral palsy. The other epileptic encephalopathies were significantly associated with malnutrition, multiplying the risk by 3.5. This association could be explained by the fact that more children with epileptic encephalopathies had cerebral palsy, and buccofacial apraxia. Likewise, we found an association between the non-West epileptic syndromes and deafness (OR 9.6) and between the other epileptic encephalopathies and buccofacial apraxia (OR 13.7). HIE multiplied the risk of malnutrition by 2.3 and multiplied the risk of mental retardation by 4.8. Unsurprisingly, HIE was associated with cerebral palsy (OR 11) and buccofacial apraxia (OR 3.8). HIE results in necrosis of the cerebral parenchyma that can lead to neurological sequelae such as cerebral palsy and favor the development of buccofacial apraxia [29,30].
The prevalence of epilepsy in Cameroon is 5.9 %. Children with epilepsy in have a mean age of 6.1 years and the main cause of the epilepsy is HIE. The majority of patients are on carbamazepine or valproate for the seizures and the epilepsies are well controlled.. Psychomotor retardation, ADHD, cerebral palsy, malnutrition and mental retardation are the most prevalent comorbidities. Cerebral palsy and mental retardation were more prevalent in children with infantile spasms, and HIE is significantly associated with buccofacial apraxia, cerebral palsy, malnutrition and mental retardation.
What is known about this topic
- The prevalence of epilepsy in sub Saharan Africa;
- The prevalence of malnutrition and mental retardation and cerebral palsy in epileptic patients;
- Etiologies of childhood epilepsy.
What this study adds
- The prevalence of attention-deficit/hyperactivity disorders in epileptic children in Africa;
- The correlation between comorbidity and epileptic syndromes;
- The correlation between comorbidity and etiologies of epilepsy.
The authors declare no competing interests.
FDT conceived and planned the study, collected data analyses and interpretation data; SN contributed in conception, interpretation of results, planned and supervise the study; NAT contributed in interpretation of results. FDN and MAA, EM, and AC were involved in planning and supervised the work. All authors provided critical feedback and helped shape the research, analysis, edited manuscript and approved the final version of the paper. All the authors have read and agreed to the final manuscript.
Table 1: sociodemographic, clinical and therapeutic characteristics of children with epilepsy at the Yaounde Gyneco-Obstetric and Pediatric Hospital, Cameroon
Table 2: correlations on the prevalence ratios of comorbidities and epileptic syndromes and comorbidities and selected etiologies
Table 3: adjusted prevalence ratios of comorbidities and epileptic syndromes and between comorbidities and some etiologies of epilepsy that showed a significant association
Figure 1: the weight-for-age curves of children with epilepsy
Figure 2: height-for-age curves of children with epilepsy
Figure 3: weight-for height curves of children with epilepsy
Figure 4: height-for-age curve of epileptic patients with epileptic encephalopathy (red line) versus non-epileptic children of the same age (green line)
Figure 5: height-for-age curve of epileptic patients without epileptic encephalopathy (red line) versus non-epileptic children of the same age (green line)
Figure 6: weight-for-age curve of epileptic patients with epileptic encephalopathy (red line) versus non-epileptic children of the same age (green line)
Figure 7: weight-for-age curve of epileptic patients without epileptic encephalopathy (red line) versus non-epileptic children of the same age (green line)
- WHO. Charge mondiale de l´épilepsie et nécessité d´une action coordonnée au niveau des pays pour influer sur ses conséquences sanitaires et sociales et sensibiliser l´opinion publique: rapport du Secrétariat. WHO Institutional Repository for Information Sharing. 2015. Accessed 9 January 2020.
- Sander JW. The epidemiology of epilepsy revisited. Curr Opin Neurol. 2003;16(2):165-170. PubMed | Google Scholar
- Mbonda E, Dongmo L, Tietche, Motso C, Sile HM, Poka D et al. Aspects cliniques et étiologiques de l´épilepsie du nourrisson et de l´enfant à Yaoundé. Curr Opin Neurol. 1995. Google Scholar
- Seidenberg M, Pulsipher DT, Hermann B. Association of epilepsy and comorbid conditions. Future Neurol. 2009 Sep 1;4(5):663-668. PubMed | Google Scholar
- Victoroff JI, Benson F, Grafton ST, Engel J, Mazziotta JC. Depression in complex partial seizures. Electroencephalography and cerebral metabolic correlates. Arch Neurol. 1994;51(2):155-163. PubMed
- Hermann BP, Seidenberg M, Bell B. Psychiatric comorbidity in chronic epilepsy: identification, consequences, and treatment of major depression. Epilepsia. 2000;41 Suppl 2:S31-41. PubMed | Google Scholar
- Crepin S, Houinato D, Nawana B, Avode GD, Preux P-M, Desport J-C. Link between Epilepsy and Malnutrition in a Rural Area of Benin. Epilepsia. 2007;48(10):1926-1933. PubMed | Google Scholar
- Sirven JI. Management of Epilepsy Comorbidities. Contin Lifelong Learn Neurol. 2016;22(1):191. PubMed | Google Scholar
- Njamnshi A, Sini V, Djientcheu V de P, Ongolo-Zogo P, Mapoure Njankouo Y, Yapnjio F et al. Risk factors associated with epilepsy in a rural area in Cameroon: A preliminary study. Afr J Neurol Sci. 2007;26(2). Google Scholar
- World Health Organization. ICD-10 Guide for Mental Retardation. ICD-10 Guide. 1996. Accessed 9 January 2020.
- Kroenke K, Spitzer RL, Williams JBW. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613. PubMed | Google Scholar
- Traoré M, Tahny R, Sacko M. Prévalence de l´épilepsie chez les enfants de 3 à 15 ans dans 2 communes du district de Bamako. Rev Neurol. 2000;156(1):1-18.
- Duggan M. Epilepsy in rural Ugandan children: seizure pattern, age of onset and associated findings. Afr Health Sci. 2010;10(3):218-225. PubMed | Google Scholar
- Koellhoffer EC, McCullough LD. The Effects of Estrogen in Ischemic Stroke. Transl Stroke Res. 2013;4(4):390-401. PubMed | Google Scholar
- Suzuki S, Brown CM, Wise PM. Neuroprotective effects of estrogens following ischemic stroke. Front Neuroendocrinol. 2009;30(2):201-211. PubMed | Google Scholar
- Murphy SJ, McCullough LD, Smith JM. Stroke in the Female: Role of Biological Sex and Estrogen. ILAR J. 2004;45(2):147-159. PubMed | Google Scholar
- Hemingway C, McGrogan J, Freeman JM. Energy requirements of spasticity. Dev Med Child Neurol. 2001;43(4):277-278. PubMed | Google Scholar
- UNICEF. Cameroon Country Profile. UNICEF Country Profile. 2019. Accessed 9 January 2020.
- UNAIDS. Cameroon Country Profile . 2019. Accessed 9 January 2020.
- Mejia AB. Pediatric Epilepsy: Diagnosis and Therapy, 3rd edition. Can J Hosp Pharm. 2009;62(3):252-253. PubMed
- Boukobza D. Évolution neuro-développementale des enfants ayant présenté un syndrome de West dans l´enfance: analyse transversale de 69 patients suivis en Haute-Normandie. Ccsd. 2016;109. Google Scholar
- Caplan R, Siddarth P, Stahl L, Lanphier E, Vona P, Gurbani S et al. Childhood absence epilepsy: behavioral, cognitive, and linguistic comorbidities. Epilepsia. 2008;49(11):1838-1846. PubMed | Google Scholar
- Ettinger AB, Weisbrot DM, Nolan EE, Gadow KD, Vitale SA, Andriola MR et al. Symptoms of depression and anxiety in pediatric epilepsy patients. Epilepsia. 1998;39(6):595-599. PubMed | Google Scholar
- Williams AE, Giust JM, Kronenberger WG, Dunn DW. Epilepsy and attention-deficit hyperactivity disorder: links, risks, and challenges. Neuropsychiatr Dis Treat. 2016;12:287-296. PubMed | Google Scholar
- Stockis A, Watanabe S, Scheen AJ. Effect of brivaracetam on CYP3A activity, measured by oral midazolam. J Clin Pharmacol. 2015;55(5):543-548. PubMed | Google Scholar
- Yates SL, Fakhoury T, Liang W, Eckhardt K, Borghs S, D´Souza J. An open-label, prospective, exploratory study of patients with epilepsy switching from levetiracetam to brivaracetam. Epilepsy Behav EB. 2015;52(Pt A):165-168.. PubMed | Google Scholar
- Quet F, Rafael F, Ngoungou EB, Diagana M, Druet-Cabanac M, Preux PM. Investigating epilepsy in Africa: 10 years of data collection using a standardized questionnaire in 2,269 peoples with epilepsy. Epilepsia. 2011;52(10):1868-1876. PubMed | Google Scholar
- Kuperminc MN, Stevenson RD. Growth and nutrition disorders in children with cerebral palsy. Dev Disabil Res Rev. 2008;14(2):137-146. PubMed | Google Scholar
- Fatemi A, Wilson MA, Johnston MV. Hypoxic Ischemic Encephalopathy in the Term Infant. Clin Perinatol. 2009;36(4):835-vii. PubMed | Google Scholar
- NORD (National Organization for Rare Disorders). Rare Disease Database. Apraxia . Accessed 9 January 2020.