Long term outcome of neonatal West Nile Virus neuroinvasive infection
Imen Bel Hadj, Ines Trabelsi, Manel Ben Romdhane, Samia Hamouda, Khadija Boussetta
Corresponding author: Imen Bel Hadj, Pediatric Department B, Children Hospital Bechir Hamza, Tunis, Tunisia 
Received: 08 May 2020 - Accepted: 18 May 2020 - Published: 02 Jun 2020
Domain: Pediatric neurology,Pediatrics (general)
Keywords: Newborn, children, encephalitis, meningoencephalitis
©Imen Bel Hadj et al. PAMJ Clinical Medicine (ISSN: 2707-2797). This is an Open Access article distributed under the terms of the Creative Commons Attribution International 4.0 License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cite this article: Imen Bel Hadj et al. Long term outcome of neonatal West Nile Virus neuroinvasive infection. PAMJ Clinical Medicine. 2020;3:36. [doi: 10.11604/pamj-cm.2020.3.36.23393]
Available online at: https://www.clinical-medicine.panafrican-med-journal.com//content/article/3/36/full
Long term outcome of neonatal West Nile Virus neuroinvasive infection
Imen Bel Hadj1,2,&, Ines Trabelsi1,2, Manel Ben Romdhane1,2, Samia Hamouda1,2, Khadija Boussetta1,2
1Pediatric Department B, Children Hospital Bechir Hamza, Tunis, Tunisia, 2University of Medicine, Tunis El Manar, Tunisia
&Corresponding author
Imen Bel Hadj, Pediatric Department B, Children Hospital Bechir Hamza, Tunis, Tunisia
West Nile virus is currently one of the most widely distributed zoonotic arbovirus in the world, progressing into epidemics in many countries. While most infected patients experience mild to no symptoms, thousands of West Nile virus-associated neuroinvasive cases, presenting as meningitis, encephalitis, or acute flaccid paralysis, have been reported, even in children. However, few neonatal cases have been described in literature. West Nile Virus neuroinvasive disease can lead to severe neurological disability or death, especially in infant. We report the first tunisian case of West Nile Virus meningoencephalitis in a newborn and its outcome.
West Nile virus (WNV) is an arbovirus of the genus Flavivirus, family Flaviviridae, which has a worldwide distribution. The virus is naturally amplified and maintained within a mosquito-bird cycle. Birds are the natural hosts of WNV acting as reservoirs, while humans are dead-end hosts, occasionally infected by a mosquito bite (Culex spp. Mosquitoes). West Nile virus (WNV) infection is more frequent in adults than in children and adolescents. In the USA, WNV infection prevalence has been estimated at 4 to 8% in children and adolescents. West Nile neuroinvasive disease (WNND) represents 30% of all reported pediatric WNV infections, as in adults [1]. However, rare cases of neonatal WNV infection have been reported. We report the first Tunisian neonatal WNND case.
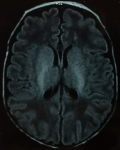
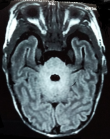
A 23 day-old boy presented at pediatric emergency with seizures. Born at term by caesearian section due to non-progression of labour, he had a birth weight of 3300g and good adaptation to extrauterine life. Since born, he was feed by breast milk and infant formula. After three days of fever (38 - 38.5oC), at age of 23 days, the newborn presented multiple episodes of myoclonic jerks and focal clonic seizures of the left hemi-body. Each seizure episode lasted for approximately two minutes, recurring every 20 to 30 minutes. On initial examination, he was healthy (weight at 3900g), aware, irritable, with a good suction, no hypotonia neither tense fontanelle. At the time of the examination, the newborn had recurrent clonic seizures of the left then the right upper limb. Seizures got controlled with intravenous phenobarbital bolus (20mg/Kg) relayed with 5mg/Kg/day of phenobarbital, without recurrence during hospitalization. Transfontanel ultrasound was normal. Biology showed negative C-reactive protein (1 mg/L) and normal blood count. Serum calcium and glucose level were normal (respectively 2.48 and 4.15 mmol/L). Cerebrospinal fluid (CSF) analysis showed a white cell count of 74x106/L, 100% lymphocytes, protein of 1.62 g/L and glucose of 2.19 mmol/L (with CSF glucose/serum glucose ratio at 53%). CSF bacterial culture showed no growth. An electroencephalogram (EEG), performed within four days after admission, was normal. Brain magnetic resonance imaging (MRI), performed 13 days after admission, revealed periventricular white matter hyperintensity associated to bilateral symmetric signal abnormalities of thalami and cerebellum white matter (Figure 1, Figure 2). These MRI anomalies were suggestive of viral encephalitis or toxic-metabolic disorders.
Ammonia serum level was normal, as was plasma amino acids and urinary organic acids chromatography. While real-time RT-PCR and PCR for herpes simplex virus, enterovirus and WNV in CSF were performed and was negative, WNV-IgM antibodies were tested positive in CSF samples. WNV meningoencephalitis was thus confirmed in view of clinical features, MRI abnormalities, positive WNV IgM antibodies in the CSF and the absence of other viral or toxic-metabolic cause. The newborn was initially treated with intravenous acyclovir. Evolution was favourable with no recurrence of seizures and normal neurological examination. Phenobarbital was discontinued after one year of follow-up with no recurrence of seizures, good psychomotor development and normal head circumference growth. Cerebral MRI at four years of follow-up revealed nodular heterotopia of the frontal periventricular grey matter, parietal and bilateral occipital grey matter. At a long term outcome of regular seven-year follow-up, our patient integrated school with good social interactions and academic performance, and has no neurological sequelae.
To date, our observation is the first Tunisian publication reporting a neonatal WNV meningoencephalitis case. Although Tunisia has experienced several WNV epidemics, the most important being those of 1968, 1970, 1997, 2003, 2007 and 2012 [2], few paediatric cases has been reported. WNV is a positive single-stranded RNA virus, transmitted to human by a mosquito bite. Human presents a very low and brief viremia [3]. Transmission of WNV through blood transfusion or solid organ transplant has been documented [4]. Transmission related to pregnancy and breastfeeding have been suggested, but all reported cases have been unconfirmed [5]. An American study showed that of 72 infants born to mothers who contracted WNV during pregnancy, only three had positive WNV-IgM antibodies, detected in cord blood. Among these newborn, only one presented encephalitis at seven days of life. The unavailability of appropriate specimens (umbilical cord, cord blood, or placenta) for confirmatory testing made impossible to proove the transplacental transmission [6]. Our patient showed clinical signs of meningoencaphalitis at the age of 23 days, breastfeeding or mosquito bite transmission cannot be determined, as mother and breast milk samples couldn´t be tested. WNV infections are asymptomatic in 80% of pediatric population, only 20% of these infected children present a febrile flu-like illness after an incubation period of 2 to 15 days. As in adults, WNND represents 30% of all reported pediatric WNV infections [4]. However, in contrast to adults, children with WNND are more likely to present with meningitis rather than encephalitis, offering a better prognosis as encephalitis lead to more severe outcomes [1]. Children with WNV encephalitis frequently presents with non-specific symptoms (fever, vomiting, headache, abdominal pain) associated to progressive altered consciousness status within one to seven days. In other cases, patient come to pediatric emergency with unresponsiveness or seizures, as it was for our patient who was hospitalized for seizures [5].
Brain MRI is important to confirm the diagnosis of encephalitis. Performed early, it may be normal. MRI may show abnormalities in the lobar grey and white matter, basal ganglia, thalami, brainstem and even cerebellum. Thalami and basal ganglia abnormalities are more often described in children, as midbrain and brainstem involvement. Meningoencephalitis imaging is nonspecific and may be confused with non-infectious processes as acute disseminated encephalomyelitis, toxic-metabolic or mitochondrial disorders or vasculitides [5]. Our patient´s MRI showed nonspecific lesions that could be related to encephalitis or toxic-metabolic disorder, normal plasma amino acids and urinary organic acids chromatography and CSF virology analysis allowed us to distinguish between these two diagnoses. Laboratory diagnosis of human WNV infection is based on WNV isolation from blood or CSF, or WNV nucleic acid detection in blood or CSF, or WNV specific antibody response (IgM) in CSF. In human viremia titers are low and often absent by the time of neurologic symptoms, making difficult direct WNV isolation and WNV nucleic acid detection [7]. WNV IgM antibodies can be detected in serum at least eight days and up to 45 days after clinical signs onset [7]. However, WNV specific antibody response is limited by a variable sensitivity of 70 to 90%, which depends on sampling time and cross-react between flaviviruses (Yellow Fever and St Louis encephalitis in particular) [4]. For our patient, detection of WNV IgM antibodies in CSF was performed four days after clinical signs onset, leading to the hypothesis of earlier positivity IgM antibodies in CSF.
Centers for Disease Control and Prevention of USA established guidelines for surveillance, prevention, and control of WNV infections in 2013. Diagnostic criteria for neuroinvasive WNV disease needs both clinical and laboratory criteria. Clinical criteria consist on fever, neurological signs of meningitis, encephalitis or acute flaccid paralysis and absence of a more likely clinical explanation. These criteria must be associated to at least one of these laboratory criteria : Isolation of virus or identification of viral antigen or nucleic acid from tissue or bodily fluid, or virus-specific IgM antibodies in CSF or serum with no other testing, or four-fold or greater increase in virus-specific quantitative antibody titers in paired sera, or virus-specific IgM antibodies in serum with confirmatory virus specific neutralizing antibodies or virus-specific IgM antibodies in CSF and a negative result for other endemic arbovirus IgM antibodies in CSF [8]. Our patient presented clinical signs and had positive WNV IgM antibodies in CSF, confirming WNND. Therapeutic management of WNND is essentially symptomatic, based on resuscitation measures for severe forms. Several therapeutic trials using intravenous immune globulin, ribavirin or a combination of them, have been carried out in adult and paediatric populations, but were inconclusive in terms of both treatment efficacy and mortality decrease [5]. West Nile neuroinvasive disease (WNND) prognosis is better in children than in adults, with a fatality rate of 1% compared to 14% in adults [9]. Long term outcome of our patient showed a favourable evolution, with no recurrence of seizures, good psychomotor development and no neurologic sequelae. Since no specific treatment is available, WNV infection prevention remains the mainstay of management. Prevention is mainly based on mosquito control through national mosquito control programs in endemic areas, as well as personal protection through repellents use on skin and/or clothes and wearing long sleeves [4]. No human vaccine is currently available; several therapeutic trials are underway with promising results [10].
West Nile neuroinvasive disease (WNND) infections are rare in children and seem exceptional in newborn. Newborn appears to be at high risk of WNV infection because of multiple probable ways of transmission: transplacental, through breast milk or mosquito bit. Despite the severity of WNND infections in adults, prognosis appears to be favourable in newborns as showed by our case report. However, prevention remains essential in pregnant women and newborns in epidemic periods.
The authors declare no competing interests.
All authors contributed to medical management of the patient presented. Article was drafting by Imen Bel Hadj (corresponding author), then revised, criticized and corrected by the other authors. The final version was approved by all authors.
Figure 1: periventricular white matter hyperintensity in brain MRI
Figure 2: bilateral and symmetric cerebellum white matter hyperintensity in brain MRI
- LaBeaud AD, Lisgaris MV, King CH, Mandalakas AM. Pediatric West Nile Virus Infection: neurologic disease presentations during the 2002 epidemic in Cuyahoga country, Ohio. Pediatr Infect Dis J. 2006 Aug;25(8):751-3. PubMed | Google Scholar
- Hammami S, Ben Hassine T, Conte A, Amdouni J, De Massis F, Sghaier S et al. West Nile disease in Tunisia: an overview of 60 years. Vet Ital. 2017;53(3):225-34. PubMed | Google Scholar
- Amdouni J, Monaco F, Portanti O, Sghaier S , Conte A, Ben Hassine T et al. Detection of enzootic circulation of a new strain of West Nile virus lineage 1 in sentinel chickens in the north of Tunisia. Acta Trop. 2020 Feb;202:105223. PubMed | Google Scholar
- Guyon G, Ladet S, Maestracci M, Jeziorski E, Foulongne V, Rodière M. West Nile Virus infections in children. Arch Pediatr. 2009 Oct;16 Suppl 2:S85-8. PubMed | Google Scholar
- Herring R, Desai N, Parnes M, Jarjour I. Pediatric West Nile Virus-Associated Neuroinvasive Disease: a Review of the Literature. Pediatr Neurol. 2019;92:16-25. PubMed | Google Scholar
- O´Leary DR, Kuhn S, Kniss KL, Hinckley AF, Rasmussen SA, Pape WJ et al. Birth outcome following WNV infection of pregnant women in the United States: 2003-2004. Pediatrics. 2006 Mar;117(3):e537-45. PubMed | Google Scholar
- Saxena V, Bolling BG, Wang T. West Nile Virus. Clin Lab Med. 2017;37(2):243-52. PubMed | Google Scholar
- Centers for Disease Control and Prevention. West Nile Virus in the United States: guidelines for surveillance. CDC. 2013.
- Lindsey NP, Hayes EB, Staples JE, Fischer M. West Nile virus disease in children, United States, 1999-2007. Pediatrics. 2009 Jun;123(6):e1084-9. PubMed | Google Scholar
- Ulbert S. West Nile virus vaccines-current situation and future directions. Hum Vaccin Immunother. 2019;15(10):2337-42. PubMed | Google Scholar