Risk-stratification and outcomes of Congolese patients with atrial fibrillation: a descriptive cohort study
Francis Beya, Cody Dinganga Malaika, Marc Tshilanda, Raissa Kuembove Kongue, Yvan Zolo, Ulrick Sidney Kanmounye
Corresponding author: Ulrick Sidney Kanmounye, Faculty of Medicine, Bel Campus University of Technology, Kinshasa, Democratic Republic of Congo 
Received: 29 Apr 2020 - Accepted: 18 May 2020 - Published: 29 Jun 2020
Domain: Cardiology
Keywords: Atrial fibrillation, Democratic Republic of Congo, diabetes, hypertension, risk assessment
©Francis Beya et al. PAMJ Clinical Medicine (ISSN: 2707-2797). This is an Open Access article distributed under the terms of the Creative Commons Attribution International 4.0 License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cite this article: Francis Beya et al. Risk-stratification and outcomes of Congolese patients with atrial fibrillation: a descriptive cohort study. PAMJ Clinical Medicine. 2020;3:75. [doi: 10.11604/pamj-cm.2020.3.75.23188]
Available online at: https://www.clinical-medicine.panafrican-med-journal.com//content/article/3/75/full
Research 
Risk-stratification and outcomes of Congolese patients with atrial fibrillation: a descriptive cohort study
Risk-stratification and outcomes of Congolese patients with atrial fibrillation: a descriptive cohort study
Francis Beya1, Cody Dinganga Malaika2, Marc Tshilanda1, Raissa Kuembove Kongue3, Yvan Zolo4, Ulrick Sidney Kanmounye3,&
&Corresponding author
Introduction: atrial fibrillation (AF) is the most common form of tachycardia. In low-income countries, the proportion of valvular AF concomitantly with rheumatic heart diseases. On the other hand, cardiovascular risk factors are increasing together with non-valvular AF. AF is responsible for 20% of strokes in patients aged 80 or older. The predicted increase in life expectancy and the epidemiologic transition in sub-Saharan Africa are expected to increase the burden of AF. This study aimed to assess the risk profile of Congolese patients with AF.
Methods: this descriptive cross-sectional study was done using data from patients admitted between 2014 and 2018 at a Congolese tertiary health facility. All AF patients admitted to the department of internal medicine during the study period were included. Measures of central tendency, hospital frequency, case fatality rate, odds ratios, and their 95% confidence intervals were calculated. The Mann-Whitney U test was used for bivariate analysis, and an alpha value of 0.05 was considered statistically significant.
Results: the hospital prevalence of AF was 0.9%. Twenty-four patients were recruited, and 54.2% were female. 83.3% of patients had a history of congestive heart failure, 37.5% had a stroke history, 37.5% had an ischemic heart disease history, 33.3% had a history of hypertension, and 25.0% had a history of diabetes. 75.0% of patients had a valvulopathy, and 70.8% had a left ventricular ejection fraction >50%. The mean CHA2DS2-VASc score was 4.3 ± 2.1, and the case fatality rate was 20.8%.
Conclusion: Congolese AF patients present a high stroke risk.
Atrial fibrillation (AF) is the most common form of tachycardia [1]. Its prevalence is between 0.4% and 2% and 4.5 million people have AF in the European Union alone [2]. In the United States, AF affects 2.3 million people, and this number is expected to rise to 15.9 million by 2050 [3]. AF-related hospitalizations have increased fourfold in 30 years in the United States [2]. The incidence of cardiovascular disease and its complications is increasing in low-income countries, especially in sub-Saharan Africa [4]. In this region, a significant cause of atrial fibrillation is valvulopathies from rheumatic heart diseases [5]. However, with the decline of rheumatic heart disease, the increasing prevalence of hypertension and the progressive aging of African populations, non-valvular atrial fibrillation has become the most frequent form of supraventricular arrhythmias [4]. Two hundred thousand Congolese have congestive heart failure [6], and 20% of these patients have AF [7]. AF accounts for 0.042% of the total burden of disease [8]. Thirty five percent of AF patients develop a stroke during their life [9], and AF is responsible for 25% of strokes in patients 80 years or older [10]. No study in the DRC has focused on the stroke risk profile of patients with AF. The aim of our work is to risk-stratify patients with AF and to describe their outcomes.
We conducted a descriptive cross-sectional study spanning five years (January 2014 to December 2018) at the Monkole Maternal and Infant Hospital Center (MMIHC). The MMHIC is a tertiary facility in Kinshasa, Democratic Republic of Congo (DRC), with a catchment area of over 2 million inhabitants [11]. We obtained approval from the institutional review board of the Faculty of Medicine, University of Our Lady of Kasayi, Kananga, DRC. We included all patients diagnosed with AF and admitted to the internal medicine department during the study period. AF was defined as irregular tachycardia (arrhythmia), of supraventricular origin, due to disorderly rapid electrical activity of the atrium (400-600/min) with loss of their hemodynamic efficiency. We collected data retrospectively from electronic health records. The data collected included patient demographics and clinical information. We calculated the measures of central tendency, hospital frequency of AF, case fatality rate, odds ratios, and their 95% confidence intervals. We used the Mann-Whitney U test to analyze CHA2DS2-VASc score differences between male and female patients, and a P-value of 0.05 was considered statistically significant. All statistical analyses were done with SPSS v26 (IBM, U.S.A.).
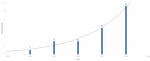
From 2014 to 2018, 24 of the 2,810 patients admitted at MMIHC´s department of Internal Medicine had an AF (hospital prevalence = 0.9%). The number of patients with AF increased exponentially from one in 2014 to eleven in 2018 (Figure 1). The patients´ mean age was 66.3 ± 13.1 years. Thirteen (54.2%) patients were women, 17 (70.8%) had a history of hypertension, and 6 (25.0%) had a history of diabetes. Twenty (83.3%) had a history of congestive heart failure, and 9 (37.5%) had a history of stroke. The most frequent presenting symptom was dyspnea (50.0%), followed by altered mental status (29.2%) (Table 1). Four (16.7%) patients had a history of both ischemic heart disease and a stroke. The odds of having ischemic heart disease and stroke were the same for both men and women (OR: 0.9, 95% CI: 0.2-4.8) (Table 2). On two-dimensional echocardiography, 18 (75.0%) patients were found to have a valvulopathy: 4 (16.7%) had single valvulopathy, 10 (41.7%) had two diseased valves, and 4 (16.7%) had three diseased valves. One (4.2%) patient had a left ventricular ejection fraction (LVEF) between 30-40%, 6 (25.0%) had an LVEF between 41-50%, and 17 (70.8%) had an LVEF >50%. The mean CHA2DS2-VASc score was 4.3 ± 2.1. Seven (29.2%) patients had a CHA2DS2-VASc score of 7, and 5 (20.8%) had a CHA2DS2-VASc score of 6 (Figure 2). The mean CHA2DS2-VASc score of female patients was 5.3 ± 2.0, while that of male patients was 3.1 ± 1.6. The CHA2DS2-VASc score difference between the two sexes was statistically significant (P = 0.009) (Figure 3). Fourteen (58.3%) patients were treated with a combination of low molecular weight heparin (LMWH), and anti-vitamin K. Five (20.8%) received anti-vitamin K alone while 2 (8.3%) were put on LMWH, anti-vitamin K, and novel oral anticoagulants. The rest received LMWH or unfractionated heparin. Eleven (45.8%) patients received beta-blockers alone, and 9 (37.5%) had beta-blockers combined with amiodarone. Five (20.8%) patients died: 2 (8.3%) had a CHA2DS2-VASc score of 2, and the other three (12.5%) had CHA2DS2-VASc scores of 4, 5, and 7. Resinusalization was achieved in 1 (4.2%) patient.
We found an AF prevalence of 0.9% in the internal medicine department. This prevalence is significantly lower compared to other studies carried out on the African continent. In Ivory Coast, the hospital prevalence was 5.5%. Senegal reported similar numbers to Ivory Coast (5.4%), but the Moroccan in-hospital frequency was at 10.8% [12]. The Ivorian, Senegalese, and Moroccan studies were all done in specialized cardiac centers. This study setting might account for the higher prevalence. Prevalence increases with age. From less than 1.0% in people aged 60 to 65 years, the prevalence of AF varies between 8.0% and 10.0% in people aged 80 or more [13]. The increase in age-related prevalence is, in part, due to an increase in the prevalence of atrial fibrillation-inducing heart diseases [3]. The mean age of Congolese AF patients was 66.3 ± 13.1 years. The mean age of the Ivorian, Senegalese, and Moroccan patients were between 58.9 and 63.0 years [5,12,14]. Hence, age might not be responsible for the difference in prevalence. There was a slight female predominance (54.2%) in our study, and female patients had higher mean CHA2DS2-VASc scores than men (5.3 ± 2.0 vs. 3.1 ± 1.6, P = 0.009). Although female patients had higher odds of congestive heart disease (OR:1.4, 95% CI: 0.2-10.0) and hypertension (OR: 1.4, 95% CI: 0.2-7.0), these were not statistically significant. AF is less prevalent among women than men, but women tend to present with atypical symptoms and to seek medical attention later [15,16]. The role of sex hormones in AF is uncertain however female patients present higher risks of AF-related complications [17-19]. No differences in the utilization of treatment and outcomes have been found between male and female patients [20] what role are more likely to develop AF. The majority of patients (70.8%) had a preserved LVEF, and a single patient had an LVEF between 30% and 40%. In the Moroccan study, 88.0% of patients had an LVEF >50%. 75.0% of patients had signs of valvular disease on echocardiography.
Hypertension is the most common risk factor associated with AF [21]; 33.3% of patients who had hypertension. In the Ivorian study, 50.2% of the patients had hypertension [14]. In Morocco and Senegal, 46.0% and 40.7% respectively had hypertension. 37.5% of patients with AF had a stroke history, and a stroke revealed an undiagnosed AF in 33.3% of cases [5,12]. The discovery of AF as a complication of a stroke suggests that there is room for improvement. Especially in the early detection and management of the risk factors of AF. AF is associated with an increased risk of stroke, heart failure, and death [21]. Also, patients with AF have a five-fold higher risk of developing a thromboembolic event [10]. AF-related thromboembolic events have an enormous financial burden [13]. AF-care related healthcare costs are higher among patients with thromboembolic events and lead to catastrophic expenditures among African patients [4]. At a system-level, AF-related healthcare costs represent 1.0% of the budget of the United Kingdom´s National Health Service and 13.5 billion euros to member states of the European Union [2]. All patients but one had a CHA2DS2-VASc score ≤2 and were anticoagulated per the 2014 AHA/ACC/HRS guidelines [22]. Fifty-eight point three percent patients were put on LMWH and anti-vitamin K while 20.8% were put on anti-vitamin K only. The choice of anticoagulant was based on the availability and affordability of the drug and its reversal agents. 45.8% of patients received a beta-blocker monotherapy, and no patients were placed on calcium channel blockers. Rhythm control does not offer a prognostic advantage over rate control [13]. Electrical cardioversion was not used either. The total case fatality rate was 20.8%. The crude mortality rate for all-cause death among patients with AF is 63.3 deaths per 1,000 patient-years [23]. Moreover, higher CHA2DS2-VASc scores, valvulopathy, hyperlipidemia, and chronic kidney disease are associated with increased mortality among AF patients [24]. Two (8.3%) of the dead patients had a CHA2DS2-VASc score of 2. Seven (29.2%) patients had a CHA2DS2-VASc score of 2. Three percent of patients in this risk-group develop a thromboembolic or mortality event [25]. The higher case fatality rate among patients with CHA2DS2-VASc score of 2 may be due to comorbidities. Limitations to our study include the lack of liver and renal function tests and the unavailability of non-pharmacologic treatments of AF. Other potential risk factors of stroke, such as smoking, were not included in this study.
Atrial fibrillation is uncommon in the MMIHC; females are most affected and presented a higher risk. Patients with AF commonly have comorbidities and are often diagnosed with AF once they have developed complications. All the patients presented at least a moderate-high risk based on the 2DS2-VASc score, and one-in-five patients died from complications of AF. Future studies should focus on the early diagnosis of AF in Congolese patients.
What is known about this topic
- Twenty percent of strokes are caused by atrial fibrillation;
- Patients with a CHA2DS2-VASc score of score are at a moderately-high risk of developing a stroke.
What this study adds
- Congolese patients with atrial fibrillation present a high risk of developing a stroke;
- One-in-five Congolese patients with atrial fibrillation die.
The authors declare no competing interests.
FB and CDM conceptualized, acquired data, administrated the project, and wrote the original draft of the manuscript. MT, RKK, and YZ validated the data analysis and wrote the original draft of the manuscript. USK analyzed the data, visualized the data outputs and wrote the original and final versions of the manuscript.
FB and CDM conceptualized, acquired data, administrated the project, and wrote the original draft of the manuscript. MT, RKK, and YZ validated the data analysis and wrote the original draft of the manuscript. USK analyzed the data, visualized the data outputs, and wrote the original and final versions of the manuscript.
Table 1: history and clinical characteristics of Congolese patients with atrial fibrillation
Table 2: the odds ratio of women versus men for comorbidities with their 95% confidence intervals
Figure 1: atrial fibrillation cases from 2014 to 2018. The gray line is a trend line with formula: number of cases = 0*exp (0.548894*Year); R-squared: 0.94; P-value: 0.007
Figure 2: CHA2DS2-VASc score distribution among Congolese patients
Figure 3: CHA2DS2-VASc score difference by sex
- Isnard R, Lacroix D. Cardiology. 2nd ed 2018 Paris, France. Elsevier Masson. Accessed 29 April 2020.
- Terrier J, Carballo S. Stratégies de dépistage de la fibrillation auriculaire. Rev Med Suisse. 2015;11(490):1892-1898.
- Morillo CA, Banerjee A, Perel P, Wood D, Jouven X. Atrial fibrillation: the current epidemic. J Geriatr Cardiol. 2017;14(3):195-203. PubMed | Google Scholar
- Mbolla BFE, Matingou AR, Ikama MS, Mongo-Ngamami SF, Landa CMK, Gombet TR et al. Coût de la prise en charge hospitalière de la fibrillation atriale non valvulaire à Brazzaville (Congo)?: étude préliminaire. Médecine et Santé Tropicales. 2016;26(2):151-153. Google Scholar
- Mbaye A, Pessinaba S, Bodian M, Ndiaye Mouhamadou B, Mbaye F, Kane A et al. Atrial fibrillation, frequency, etiologic factors, evolution and treatment in a Cardiology Department in Dakar, Senegal. Pan Afr Med J. 2010;6:16. PubMed | Google Scholar
- Kaur R, Isherwood A, Ayodele L, Hughes M. P2452 The global burden of chronic heart failure. Eur Heart J. 2017;38(suppl-1). Google Scholar
- Malamba-Lez D, Ngoy-Nkulu D, Steels P, Tshala-Katumbay D, Mullens W. Heart failure etiologies and challenges to care in the developing world: an observational study in the Democratic Republic of Congo. Journal of Cardiac Failure. 2018;24(12):854-859. PubMed | Google Scholar
- 17. Institute for health metrics and evaluation. Global burden of disease visualization tool. Accessed 28 April 2020.
- Blackshear JL, Odell JA. Appendage obliteration to reduce stroke in cardiac surgical patients with atrial fibrillation. Ann Thorac Surg. 1996;61(2):755-759. PubMed | Google Scholar
- National institute of neurological disorders and stroke. Atrial fibrillation and stroke. 2019. Accessed 28 April 2020.
- Présentation du Centre Hospitalier Mère et Enfant Monkole. Presentation de monkole.. Accessed 20 October 2019.
- Dannouni Y. Complete arrhythmia by atrial fibrillation: etiologies and management. 2015. Accessed 29 April 2020.
- Nesheiwat Z, Goyal A, Jagtap M. Atrial Fibrillation (A Fib). In: StatPearls 2020 Treasure Island (FL) StatPearls Publishing. 2020. PubMed
- Coulibaly I, Anzouan-Kacou J, Kouao Konin C, Kouadio S, Abouou N. Atrial fibrillation: epidemiology at the cardiology institute of Abidjan (Ivory Coast). Med Trop. 2010;70(4):371-374. PubMed | Google Scholar
- Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin EJ et al. Worldwide epidemiology of atrial fibrillation: a global burden of disease 2010 study. Circulation. 2014;129(8):837-847. PubMed | Google Scholar
- Ball J, Carrington MJ, Wood KA, Stewart S. Women Versus Men with Chronic Atrial Fibrillation: Insights from the Standard Versus Atrial Fibrillation spEcific managemenT studY (SAFETY). PLoS One. 2013 May 29;8(5):e65795. PubMed | Google Scholar
- Ko D, Rahman F, Schnabel RB, Yin X, Benjamin EJ, Christophersen IE. Atrial fibrillation in women: epidemiology, pathophysiology, presentation, and prognosis. Nat Rev Cardiol. 2016;13(6):321-332. PubMed | Google Scholar
- Bekwelem W, Connolly SJ, Halperin JL, Adabag S, Duval S, Chrolavicius S et al. Extracranial systemic embolic events in patients with nonvalvular atrial fibrillation: incidence, risk factors, and outcomes. Circulation. 2015;132(9):796-803. PubMed | Google Scholar
- Bushnell C, McCullough LD, Awad IA, Chireau MV, Fedder WN, Furie KL et al. Guidelines for the prevention of stroke in women: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(5):1545-1588. PubMed | Google Scholar
- Ko D, Rahman F, Martins MAP, Hylek EM, Ellinor PT, Schnabel RB et al. Atrial fibrillation in women: treatment. Nat Rev Cardiol. 2017;14(2):113-124. PubMed | Google Scholar
- Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37(38):2893-2962. PubMed | Google Scholar
- January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation. 2014 Dec 2;130(23):e199-267. PubMed | Google Scholar
- Lee E, Choi EK, Han KD, Lee H, Choe WS, Lee SR et al. Mortality and causes of death in patients with atrial fibrillation: a nationwide population-based study. PLoS One. 2018;13(12). PubMed | Google Scholar
- Gažová A, Leddy JJ, Rexová M, Hlivák P, Hatala R, Kyselovic J. Predictive value of CHA2DS2-VASc scores regarding the risk of stroke and all-cause mortality in patients with atrial fibrillation (CONSORT compliant). Medicine (Baltimore). 2019;98(31):e16560. PubMed
- Parsons C, Patel SI, Cha S, Shen W-K, Desai S, Chamberlain AM et al. CHA2DS2-VASc Score: a predictor of thromboembolic events and mortality in patients with an implantable monitoring device without atrial fibrillation. Mayo Clin Proc. 2017;92(3):360-369. PubMed | Google Scholar