Feto-maternal complications and outcomes of pregnant women with valvular heart disease in a tertiary center in Ethiopia
Moges Beriye, Fitsum Araya, Yesuf Ahmed, Elias Ali, Elsah Tegene, Tesfamicheal Alaro, Muktar Beshir, Abdi Befikadu, Hiwot Amare
Corresponding author: Hiwot Amare, Department of Internal Medicine, Institute of Health, Jimma University, Jimma, Ethiopia 
Received: 17 Mar 2021 - Accepted: 29 Apr 2021 - Published: 05 May 2021
Domain: Cardiology,Obstetrics and gynecology,Maternal and child health
Keywords: Ethiopia, feto-maternal complications, pregnancy, rheumatic heart disease, valvular heart disease
©Moges Beriye et al. PAMJ Clinical Medicine (ISSN: 2707-2797). This is an Open Access article distributed under the terms of the Creative Commons Attribution International 4.0 License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cite this article: Moges Beriye et al. Feto-maternal complications and outcomes of pregnant women with valvular heart disease in a tertiary center in Ethiopia. PAMJ Clinical Medicine. 2021;6:5. [doi: 10.11604/pamj-cm.2021.6.5.28920]
Available online at: https://www.clinical-medicine.panafrican-med-journal.com//content/article/6/5/full
Research 
Feto-maternal complications and outcomes of pregnant women with valvular heart disease in a tertiary center in Ethiopia
Feto-maternal complications and outcomes of pregnant women with valvular heart disease in a Tertiary Center in Ethiopia
Moges Beriye1, Fitsum Araya1, Yesuf Ahmed1, Elias Ali2, ![]() Elsah Tegene3, Tesfamicheal Alaro2,
Elsah Tegene3, Tesfamicheal Alaro2, ![]() Muktar Beshir4, Abdi Befikadu5,
Muktar Beshir4, Abdi Befikadu5, ![]() Hiwot Amare3,&
Hiwot Amare3,&
&Corresponding author
Introduction: globally, valvular heart disease (VHD) is a major cause of heart disease and is associated with indirect maternal mortality. Pregnancy associated with VHD results in various feto-maternal complications. The objective of this study was to evaluate the maternal and fetal complications and outcomes of pregnancies complicated by VHD at Jimma University medical center.
Methods: a hospital-based cross-sectional study was conducted among all pregnant mothers with VHD that visited Jimma University Medical center, from September 1, 2018 to July 30, 2019.
Results: the prevalence of VHD in pregnant women was 0.6%. Out of a total of 29 pregnant women 28 (96.6%) had rheumatic heart disease. Mitral stenosis (75.9%) was the most frequent mitral valve pathology and 16 (55.2%) had severe mitral stenosis. The commonest maternal complication was new onset or worsening of heart failure in 22 (75.9%). The commonest neonatal complication was preterm delivery (39.3%). There were 2 postpartum maternal deaths (6.9%) and 4 (14.3%) early neonatal deaths.
Conclusion: majority of pregnant women with VHD had rheumatic heart disease. Adverse feto-maternal complications were common. Expanding access to cardiovascular interventions and feto-maternal medicine services is recommended.
Pregnancy results in physiologic changes in the cardiovascular diseases [1,2]. Cardiac output rises during pregnancy with the highest increase in the first and second trimester [3]. Pregnancy is associated with vasodilation [1] and peripheral vascular resistance decreased [4]. Heart rate and cardiac contractility is also increased [5]. Cardiac diseases are classified as congenital or acquired [6]. Congenital heart diseases (CHD) are further classified as cyanotic or acyanotic congenital heart diseases [6]. Whereas, the acquired heart diseases are categorized into coronary artery diseases, valvular heart diseases (VHD), hypertensive heart disease and cardiomyopathies and others. Heart disease complicates pregnancy in 1-3% of cases [7]. Heart disease is one of the major causes of indirect maternal mortality and causes 10-25% of maternal mortality [8]. Valvular heart disease complicating pregnancy is increasing [9]. In pregnancy, stenotic valvular lesions are not well tolerated as compared to regurgitant lesions [9].
Globally, VHD is a major cause of heart disease [10]. In low middle-income countries, rheumatic heart disease (RHD) is the commonest cause of VHD [11]. Congenital and degenerative VHD are commonest etiology of VHD in developed countries [9,11]. Congenital VHD such as bicuspid aortic valve, mitral valve prolapse and pulmonary stenosis are also other causes of VHD [12,13]. Pregnancy associated with VHD results in various feto-maternal complications [14]. Maternal and fetal complications include congestive heart failure, arrhythmia, pulmonary edema, pre-eclampsia, preterm delivery, low birth weight (LBW), intrauterine growth restriction, fetal loss, neonatal mortality and maternal mortality [15]. In settings like Ethiopia, access to invasive cardiac procedures is poor and cardiac surgery is mainly performed in humanitarian campaigns. This results in suboptimal treatment of structural heart diseases including VHD. Therefore, we expect pregnant women to have more fetal and maternal complications in this setting. But these complications have not been studied. Therefore, we studied maternal and fetal complications and pregnancy of women having VHD.
A hospital-based cross-sectional study was conducted at Jimma University medical center (JUMC), in Jimma town, Ethiopia from September 1, 2018 to July 30, 2019. All pregnant women with VHD managed at JUMC during the study period were included. All cases that have proven pregnancy, any type of VHD confirmed by echocardiography that provided written informed consent were included. Those with incomplete data documentation or who did not consent were excluded. Data was collected using a structured data questionnaire written in English from patients or attendants and patients´ charts. After detailed training on the data collection instrument was given, physicians collected data and completed questionnaire. Two-dimensional trans-thoracic echocardiography was used to diagnose cardiac disease and was performed according to American Society of Echocardiography Guideline [16]. Valve lesions were graded according to European Society of Cardiology guideline [17]. Pulmonary hypertension was diagnosed and graded according to European Society of Cardiology guideline [18]. Rheumatic heart disease was diagnosed based on the World Heart Federation criteria [19]. Atrial fibrillation was diagnosed by 12-lead electrocardiography according to American Heart Association criteria [20]. New York Heart Association (NYHA) classification was used to assess functional status of heart failure patients [21].
The main variables of interest were feto-maternal complications and outcomes. Maternal outcomes included development or worsening of congestive heart failure, pulmonary edema, persistent atrial fibrillation, cardiogenic shock, cardiac arrest, need for ventilation, admission to intensive care unit, change in NYHA functional status, stroke, infective endocarditis, pregnancy induced hypertension, preterm premature rupture of membrane, cesarean delivery, postpartum hemorrhage, sepsis and maternal death. Fetal outcomes included preterm birth, termination of pregnancy for cardiac indication, still birth, early neonatal death, need for neonatal intensive care unit admission, birth weight and low fifth minute APGAR score. Cardiac Disease in Pregnancy (CARPREG) score was calculated by giving one point for each of the following parameters: previous cardiac event (heart failure, transient ischemic attack, or stroke before pregnancy), initial NYHA functional class > II or cyanosis, left heart obstruction (mitral valve area < 2cm2 or aortic valve area <1.5cm2 or peak left ventricular outflow tract gradient >30 mmHg) and reduced left ventricular systolic function (ejection fraction < 40%) [22]. The sum of predictor points (maximum scores of four) was used to predict maternal and neonatal events [22].
Maternal death was defined as death of mother during pregnancy, delivery or 42 days postpartum irrespective of site or duration of pregnancy which is directly or indirectly related to the pregnancy. Pulmonary edema was diagnosed if chest radiograph shows evidence or if physical examination showed crackles heard over more than one-third of posterior lung fields. If delivery occurred before 37 completed weeks of pregnancy preterm delivery was considered [23]. Small for gestational age (SGA) is birth weight centiles of less than 10th centile [24]. Premature rupture of membrane (PROM) was defined as rupture of membrane before onset of labor [25]. Intrauterine growth retardation-fetal/newborn was defined as weight less than 10th centile for its gestational age (GA) [26]. Low birth weight (LBW) was defined birth weight < 2500 grams [27]. Early onset neonatal sepsis was defined as neonatal sepsis until seven days of delivery [28]. Still birth was defined as death of fetus before delivery which can be either before onset of labor or intrapartum [29]. Abortion was defined as loss of pregnancy before 28 weeks of gestation [30]. Early neonatal death was considered as death of a newborn in the first seven days [31]. The data collection was supervised by the principal investigator and inaccurate and incomplete data was cleaned before analysis. Epidata version 3.1 was used for data entry and STATA version 12.0 used for data analysis. Descriptive Data analysis was performed.
Ethical issues and informed consent: ethical clearance was obtained from Institutional Review Board of Jimma University Institute of health (IHRGPD/206/17). Written informed consent was obtained from all women. Confidentiality of information collected from each study participant was maintained.
During the study period, a total of 4584 women delivered at JUMC out of which 29 (0.6%) women had proven VHD. Twenty women (69%) were included to the study during pre-partum period and the rest were included after onset of labor. The mean (±SD) age was 29 (±6) years (Table 1). Majority of patients (89.7%) had history of counseling on contraception. Twenty (69%) were using either artificial method (Injectable 13 (44.8%) and Implanon 4 (13.8%) or natural method (calendar 3 (10.3%). Nearly all women 27 (93.1%) had no pre-conceptional counseling. Only nine (31%) index pregnancies were planned. Majority were gravida II to IV 12 (41.4%), 11 (37.9%) were gravida V or more and 6 (20.7%) were primigravida. Fifteen women (51.7%) were having four to seven antenatal care (ANC) visits. Nine women (31%) had ANC visits of three or less. Twenty-one women (72.4%) visited JUMC after they have reached the third trimester, either before onset of labor or intrapartum. Sixteen women (55.2%) were para I to IV. Sixteen (55.1%) women were diagnosed with VHD during pregnancy. Ten (34.5%), 5 (17.2%) and 1(3.4%) were diagnosed during third, second and first trimester respectively. Thirteen women (44.8 %) have been diagnosed with VHD prior to the index pregnancy. Almost all (96.6%) had RHD as an etiology of VHD except one who was diagnosed with congenital valve disease. None had other known chronic medical illness or family history of known chronic medical illness. The duration of disease was less than five years in ten (76.8%) women.
On medical history, 27 (93.1%) had shortness of breath, 22 (75.9%) had paroxysmal nocturnal dyspnea, 20 (69.0%) had palpitation, 18 (64.3%) had body swelling, 6 (21.4%) had chest pain, 1 (3.5%) had cyanosis, 1 (3.5%) had presyncope and 1 (3.5%) had syncope. Two (7.1%) women had past limb paralysis with deviation of mouth and one had past history of difficulty of speech that resolved by itself. Before index pregnancy, 10 (34.5%) were taking diuretics, 6 (20.7%) were taking angiotensin converting enzyme inhibitors and only 6 (46.2%) were taking monthly intramuscular benzathine penicillin. Five (17.2%) women were taking β-blockers whereas 3 (10.3%) were taking digoxin and 2 (6.9%) were taking anticoagulants. None had history of cardiac surgery. At initial visit, 12 (41.4%) were in NYHA class I and II and 17 (58.6%) were in NYHA class III & IV. Majority (65.5%) had left ventricular dysfunction out of which 6 (20.7%) had severe ventricular dysfunction. Twelve (41.4%) had pulmonary hypertension in which mild, moderate and severe pulmonary hypertension each accounting for 4 (13.8%) cases (Table 2). On electrocardiogram, nine (34.5%) women had atrial fibrillation before or after delivery, one (3.4%) had left axis deviation and one (3.4%) had left atrial enlargement.
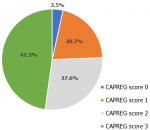
The commonest valve affected by VHD was mitral valve 26 (89.7%) followed by aortic 16 (55.2%) and tricuspid 13 (44.8%) valves. Isolated mitral valve lesion was found in four (13.9%) women and two had severe mitral stenosis (MS). Majority (51.7%) had either isolated or combined mitral and aortic valves lesions only. Combined MS with MR (mitral regurgitation) and aortic regurgitation (AR) was the commonest mixed valve abnormality (13.8%) followed by combined MS with tricuspid regurgitation (TR) (10.3%). From twenty-two (75.9%) that had MS, 16 (55.2%) women had severe MS. MR was found in 17 (58.6%) women out of which 10 (34.5%) had moderate MR, 5 (17.2%) had mild MR and 2 (6.9%) had severe MR. Only 3 (10.3%) had AS (aortic stenosis) in which 2 (6.9%) mild AS and 1 (3.4%) moderate AS. AR was found in 13 (45.8%) women out of which 7 (24.1%) had moderate AR, 3 (10.3%) had mild AR and 3 (10.3%) had severe AR. TR was found in 13 (44.8%) of which 5 (17.2%), 5 (17.2%) & 3 (10.3%) had severe, mild and moderate TR respectively. Only one (3.4%) had moderate tricuspid stenosis (TS). Pulmonary regurgitation (PR) and pulmonary stenosis (PS) were found only in 3 (10.3%) cases. From mixed valvular lesions, 4 (13.8) women had MS+MR+AR. MS+MR+TR, MS+AR+TR, MS+MR, MR+AR, MS+MR+AR+TR were identified in 2 (6.9) women correspondingly. MS+AR, MS+MR+AS, MS+AS+TR, AS+PR+TR and MS+MR+TR+PR+TS were identified in 1 (3.4%) woman each respectively. Majority (47.3%) had CARPREG score of 3 and 27.6% had CARPREG score of 2 (Figure 1).
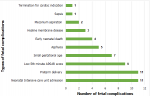
Seventeen women (85%) had either primarily obstetrics or primarily cardiac adverse maternal outcomes. Adverse maternal cardiac outcomes were identified in 89.7% of cases. Majority had new or worsening of heart failure (75.9%). During the follow up, five (17.2%) women experienced deterioration of functional status. Two maternal deaths (6.9%) occurred, both during post-partum period. In both cases, cause of maternal death was cardiogenic shock which complicated by cardiac arrest. Out of 29 pregnancies, 28 (96.4%) resulted in live birth and one ended up with therapeutic abortion at first trimester for indication of severe MS. The mean GA at end of pregnancies were 35.9 (±5.8) weeks. Labor onset was spontaneous in 18 (64.3%) mothers. Fourteen women (48.3%) had instrument delivery (either vacuum or forceps) and five (17.2%) gave birth by cesarean section. Ten (35.7%) mothers had induced labor. The indication for inducing labor were full term pregnancy with heart disease in 6 (21.5%), pre-eclampsia in 3 (10.7%) and PROM with chorioamnionitis in 1 (3.5%). Two of pregnancy induced for pre-eclampsia are preterm making iatrogenic preterm delivery 3 (10.7%). From pregnancies which reached age of viability (≥28 weeks), preterm delivery occurred in 11 (39.3%) women. Fetal outcomes were calculated out of pregnancy reached GA of ≥28 weeks. The mean birth weight of neonates was 2640 (±690) grams ranging from 1200 to 3900 grams. Eleven (39.3%) neonates were admitted to neonatal intensive care unit with diagnosis. Ten (35.7%) newborns had LBW. Seven (25%) newborns were SGA with mean weight of 1921(±565) grams. Fifth minute APGAR score were low in 9 (32.1%) newborns (Figure 2).
Among pregnant women with VHD at a tertiary center in Ethiopia, majority had RHD and MS was the commonest valve lesion. More than half were in either NYHA class III or IV heart failure and had left ventricular dysfunction. Most pregnant women had adverse maternal outcomes. New or worsening of heart failure was the commonest cardiac complication. Almost half had instrumental delivery. Neonatal intensive care unit (NICU) admission for various reasons and LBW were common neonatal complications. A study among Indian pregnant women had RHD (88%) and mitral valve was the commonest valve involved [32]. Rheumatic heart disease (66%) and CHD (25.7%) were top two causes of heart disease in pregnant women in Thailand [33]. This is in line with our study. Congenital heart diseases contributed about 13% of cases in the Indian study. This is higher as compared to the finding our study and it could be due to the small number of cases in our study. In low income and middle-income countries, RHD is common in pregnant women and the general population. But in developed countries the prevalence of RHD has decreased and is currently low in the general population. The Registry of Pregnancy and Cardiac Disease (ROPAC) sub study, which assessed pregnant women with structural heart disease showed 70% had mitral valve disease (MS and/or MR). We found that 90% of our participants had mitral valve diseases and this could be due to high prevalence of RHD among our participants and in Ethiopian population [34] and in Jimma zone as reported by Gemechu et al. [35]. Left ventricular systolic dysfunction was identified in 13.9% of pregnant Pakistani women [36]. In contrast, our participants had high left ventricular systolic dysfunction (65.5.%).
Half of our participants were in NYHA class IV heart failure. This is higher as compared to studies from Thailand [33]. During follow up, new or worsening of heart failure was found in 75.9% and 17.2% of experienced deterioration in functional status. During follow-up, 20% of pregnant Nepalese women with valvular heart disease experienced deterioration of NYHA functional class [37] which is slightly higher than our study. This could be due to the absence of surgical treatment of VHD among our participants. Majority of women had live birth and one ended up with therapeutic abortion at first trimester for indication of severe MS. Similar findings of neonatal death were identified in a study from Thailand [33]. At end of pregnancies, the mean GA were 35.9 (±5.8) weeks. Similar findings were found in a study from South Africa [38] and slightly higher GA was seen in studies from Nepal [39] and Netherland [40]. Valvular heart diseases in pregnant women increases premature delivery [41]. There are inconclusive reports concerning prevalence of premature delivery among pregnant women with heart disease. Two studies from Pakistan on pregnant women with MS reported preterm delivery ranged from 17% to 21.8% [36,42]. In studies from Thailand, Egypt and Nepal premature delivery was reported to be 16.5%, 34.3% and 22.4% respectively [33,37,43]. Our study reported higher prevalence of preterm delivery (39.3%).
Pregnant Egyptian women with VHD reported a mean birth weight of neonates to be 2635.7± 656.7 gram which is similar to our findings [43]. However, studies from developed countries reported higher neonatal weight as compared to our study [40,41]. As many participants from developed countries had NYHA class I/II and this could result in improved neonatal weight at delivery as functional class affect perinatal outcomes [32,40,41]. A study from Netherland showed SGA was seen in 21.5% and which is a slightly lower prevalence but similar average weight of SGA neonates to our findings [40]. The Netherland study also reported 21.8% of neonates were admitted to NICU which is lower than the result reported in our study. This could be due to low pre-conceptional counseling, worse functional status among our study participants. Low birth weight is one of the common complications of pregnancy with heart disease. Ten (35.7%) newborns had LBW. In Pakistani pregnant women with heart disease LBW was seen in 45% [44]. The ROPAC study reported a LBW rate of 16.4% [45]. In India pregnant women with heart disease, LBW was 32.5% [46]. Among Pakistani pregnant women with mitral stenosis low APGAR score in neonates was 14.9% [36]. Our study has higher prevalence of low fifth-minute APGAR score (32.1%) newborns. Many of our participants had higher NYHA class in our study which could increase neonatal complications [32,47]. Nine had intra uterine fetal death (IUFD) in the Pakistani study which included women with isolated MS [36]. Intra uterine fetal death was reported in eight pregnancies in Nepal [37]. There was no IUFD was reported in our study. This disparity could be due the difference in inclusion criteria between these studies. Isolated MS could be associated with high adverse fetal outcomes. The difference in sample size could also contribute to the disparity in occurrence of IUFD.
Cardiovascular Diseases (CVDs) in pregnancy are one the commonest indirect causes of maternal morbidity and mortality [48]. Studies concerning CVDs in pregnant women from developing countries show high adverse maternal outcomes. High adverse maternal outcomes have been identified in our study. As none of our participants had surgical interventions for their valvular lesions, this could increase adverse maternal outcomes [32]. Our participants had high prevalence of pulmonary hypertension which is known to be associated with adverse maternal events [22]. Similar maternal mortality was found in the Pakistani study and in our study [36]. In a tertiary hospital, 34% of Senegalese pregnant women with CVDs have died [49]. Though there are no strong studies in multivalvular heart disease, it has been reported that high-risk of complications were associated with multivalvular heart disease with stenotic valve disease [47]. High maternal adverse events could be due to high rate of multivalvular heart disease with predominant MS among our participants. Our study has various limitations. We couldn´t test for associations as our study has small samples size. Echocardiography was interpreted by one technician and this could introduce bias.
We showed that majority of pregnant women with VHD had rheumatic heart disease. Mitral stenosis was the commonest valve lesions. More than half were in either NYHA class III or IV heart failure and had ventricular dysfunction. Adverse feto-maternal complications were common. Improved access to cardiovascular interventions such as valve repair and replacement is recommended. Expansion of feto-maternal medicine services in Ethiopia is recommended to improve neonatal and maternal outcomes and reduce indirect maternal mortality.
Funding sources: the study was funded by Jimma University Institute of Health research fund.
What is known about this topic
- Cardiovascular diseases complicate pregnancy and are indirect cause of maternal mortality;
- In countries such as Ethiopia, access to cardiac surgery is poor and women with cardiac disease are less likely to get interventional CVD treatments.
What this study adds
- Rheumatic heart disease with predominant mitral stenosis was commonest VHD;
- New or worsening of heart failure in pregnant women and NICU admission were ubiquitous complications. Moreover, improving care for CVDs could further improve outcomes.
The authors declare no competing interests.
MB, HA, FA and YA conceived the study. MB, FA, HA and YA collected the data. MB, EA, TA, ET, AB and MB analyzed the data. MB drafted the manuscript and HA, EY, FA commented on the first draft of the manuscript. All authors confirmed the final version of the manuscript.
We would like to thank our study participants and Jimma University for providing the set up for the research.
Table 1: socio-demographic characteristics of pregnant women with valvular heart diseases at Jimma University medical center (JUMC), in Jimma town, Ethiopia from September 1, 2018, to July 30, 2019
Table 2: medical history of pregnant women with valvular heart diseases at Jimma University Medical Center (JUMC), in Jimma town, Ethiopia from September 1, 2018, to July 30, 2019
Figure 1: cardiac disease in pregnancy (CARPREG) score of pregnant women with valvular heart diseases at Jimma University Medical Center (JUMC), in Jimma town, Ethiopia from September 1, 2018, to July 30, 2019
Figure 2: fetal and neonatal outcomes of pregnancies with valvular heart diseases at Jimma University Medical Center (JUMC), in Jimma town, Ethiopia from September 1, 2018, to July 30, 2019
- Chapman AB, Abraham WT, Zamudio S, Coffin C, Merouani A, Young D et al. Temporal relationships between hormonal and hemodynamic changes in early human pregnancy. Kidney International. 1998;54(6):2056-2063. PubMed | Google Scholar
- Sanghavi M, Rutherford JD. Cardiovascular physiology of pregnancy. Circulation. 2014;130(12):1003-1008. PubMed | Google Scholar
- Bader RA, Bader ME, Rose DF, Braunwald E. Hemodynamics at rest and during exercise in normal pregnancy as studied by cardiac catheterization. The Journal of Clinical Investigation. 1955;34(10):1524-1536. PubMed | Google Scholar
- Mahendru AA, Everett TR, Wilkinson IB, Lees CC, McEniery CM. A longitudinal study of maternal cardiovascular function from preconception to the postpartum period. Journal of Hypertension. 2014;32(4):849-856. PubMed | Google Scholar
- Clapp III JF, Capeless E. Cardiovascular function before, during, and after the first and subsequent pregnancies. The American journal of cardiology. 1997;80(11):1469-1473. PubMed | Google Scholar
- Gei AF, Hankins GDV. Cardiac disease and pregnancy. Obstetrics and Gynecology Clinics of North America. 2001;28(3):465-512. PubMed | Google Scholar
- Arafeh JM, Baird SM. Cardiac disease in pregnancy. Critical Care Nursing Quarterly. 2006;29(1):32-52. PubMed | Google Scholar
- Dobbenga-Rhodes YA, Privé AM. Assessment and evaluation of the woman with cardiac disease during pregnancy. The Journal of Perinatal & Neonatal Nursing. 2006;20(4):295-302. PubMed | Google Scholar
- Goldstein SA, Ward CC. Congenital and acquired valvular heart disease in pregnancy. Current Cardiology Reports. 2017;19(10):96. PubMed | Google Scholar
- Maganti K, Rigolin VH, Sarano ME, Bonow RO. Valvular heart disease: diagnosis and management. Mayo Clin Proc. 2010 May;85(5):483-500. PubMed | Google Scholar
- Bernard Iung, Alec Vahanian. Epidemiology of valvular heart disease in the adult. Nature Reviews Cardiology. 2011;8(3):162-172. Google Scholar
- LaHaye S, Lincoln J, Garg V. Genetics of valvular heart disease. Current Cardiology Reports. 2014;16(6):487. PubMed | Google Scholar
- Rose AG. Etiology of valvular heart disease. Current opinion in cardiology. 1997;11(2):98-113. PubMed | Google Scholar
- Roos-Hesselink J, Baris L, Johnson M, De Backer J, Otto C, Marelli A et al. Pregnancy outcomes in women with cardiovascular disease: evolving trends over 10 years in the ESC registry of pregnancy and cardiac disease (ROPAC). European Heart Journal. 2019;40(47):3848-3855. PubMed | Google Scholar
- Hameed A, Karaalp IS, Tummala PP, Wani OR, Canetti M, Akhter MW et al. The effect of valvular heart disease on maternal and fetal outcome of pregnancy. Journal of the American College of Cardiology. 2001 Mar 1;37(3):893-9. PubMed | Google Scholar
- Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography´s Guidelines and Standards Committee and the Chamber Quantification Writing Group, Developed in Conjunction with the European Association of Echocardiography, a Branch of the European Society of Cardiology. Journal of American Society of Echocardiography. 2005 Dec;18(12):1440-63. PubMed | Google Scholar
- Baumgartner H. 2017 ESC/EACTS guidelines for the management of valvular heart disease. European heart journal. 2017 Sep 21;38(36):2739-2791. PubMed | Google Scholar
- Sirnes PA. Guidelines for the diagnosis and treatment of pulmonary hypertension. European Heart Journal. 2009 Oct;30(20):2493-537. PubMed | Google Scholar
- Reményi B, Wilson N, Steer A, Ferreira B, Kado J, Kumar K, Lawrenson J. World heart federation criteria for echocardiographic diagnosis of rheumatic heart disease-an evidence-based guideline. Nature Reviews Cardiology. 2012;9(5):297-309. PubMed | Google Scholar
- Estes NAM. ACC/AHA/physician consortium 2008 clinical performance measures for adults with nonvalvular atrial fibrillation or atrial flutter. Circulation. 2008;117(8):1101-1120. PubMed | Google Scholar
- Hurst JW, Morris DC, Alexander RC. The use of the New York Heart Association's classification of cardiovascular disease as part of the patient's complete problem list. Clinical cardiology. 1999;22(6):385-390. PubMed | Google Scholar
- Silversides CK. Pregnancy outcomes in women with heart disease CAPREG. Journal of the American College of Cardiology. 2018;71(21):2419-2430. PubMed | Google Scholar
- Vogel JP, Chawanpaiboon S, Watananirun K, Lumbiganon P, Petzold M, Moller AB et al. Global, regional and national levels and trends of preterm birth rates for 1990 to 2014: protocol for development of World Health Organization estimates. Reproductive Health. 2016;13(1):76. PubMed | Google Scholar
- McCowan L, Horgan RP. Risk factors for small for gestational age infants. Best Practice & Research Clinical Obstetrics & Gynaecology. 2009;23(6):779-793. PubMed | Google Scholar
- Tigist Endale, Netsanet Fentahun, Desta Gemada, Mamusha Aman Hussen. Maternal and fetal outcomes in term premature rupture of membrane. World journal of emergency medicine. 2016;7(2):147-52. PubMed | Google Scholar
- Wollmann HA. Intrauterine growth restriction: definition and etiology. Hormone Research in Paediatrics. 1998;49(Suppl 2):1-6. PubMed | Google Scholar
- World Health Organization. Global nutrition targets 2025: Low birth weight policy brief. World Health Organization. 2014. Google Scholar
- Edwards MS, Baker CJ. Krugman´s infectious diseases of children. Sepsis in the newborn. 2004;11:545-561.
- Da Silva FT. Stillbirth: case definition and guidelines for data collection, analysis, and presentation of maternal immunization safety data. Vaccine. 2016 Dec 1;34(49):6057-6068. PubMed | Google Scholar
- Cunningham F. Overview of obstetrics, Williams obstetrics. 23rd ed New York: McGraw-Hill. 2010.
- Lehtonen L. Early neonatal death: a challenge worldwide; in seminars in fetal and neonatal medicine. Elsevier. 2017. Google Scholar
- Bhatla N, Lal S, Behera G, Kriplani A, Mittal S, Agarwal N et al. Cardiac disease in pregnancy. International Journal of Gynecology and Obstetrics. 2003;82(2):153-159. PubMed | Google Scholar
- Chumpathong S, Sirithaweesit C, Pechpaisit N, Suraseranivongse S, von Bormann B, Titapant V et al. Predictors for complications in pregnant women with heart disease, a retrospective study. J Med Assoc Thai. 2014 Jul;97(7):730-5. PubMed | Google Scholar
- Engel ME, Haileamlak A, Zühlke L, Lemmer CE, Nkepu S, van de Wall M et al. Prevalence of rheumatic heart disease in 4720 asymptomatic scholars from South Africa and Ethiopia. Heart. 2015;101(17):1389-1394. PubMed | Google Scholar
- Gemechu T, Mahmoud H, Parry EH, Phillips DI, Yacoub MH. Community-based prevalence study of rheumatic heart disease in rural Ethiopia. European Journal of Preventive Cardiology. 2017;24(7):717-723. PubMed | Google Scholar
- Ahmed N, Kausar H, Ali L, Rakhshinda. Fetomaternal outcome of pregnancy with Mitral stenosis. Pakistan Journal of Medical Sciences. 2015;31(3):643. PubMed | Google Scholar
- Koirala PS. Obstetric outcome in patients with rheumatic heart disease: experience of a tertiary hospital. Nepalese Heart Journal. 2017;14(2):31-34. Google Scholar
- Makgato C, Baloyi S, Nondabula T. Profile of cardiac patients who delivered at Universitas Academic Hospital (UAH) in Bloemfontein South Africa: 2012-2017; in Obstetrics and Gynaecology Forum. In House Publications. 2020;30(2). Google Scholar
- Paudyal P, Rawal S. Fate of pregnancy in women with rheumatic heart disease attending a tertiary referral centres. JNDA. 2017;7(2). Google Scholar
- Hink, E, Bolte AC. Pregnancy outcomes in women with heart disease: experience of a tertiary center in the Netherlands. Pregnancy Hypertension: An International Journal of Women's Cardiovascular Health. 2015;5(2):165-170. PubMed | Google Scholar
- Hameed A, Karaalp IS, Tummala PP, Wani OR, Canetti M, Akhter MW et al. The effect of valvular heart disease on maternal and fetal outcome of pregnancy. Journal of the American College of Cardiology. 2001;37(3):893-899. PubMed | Google Scholar
- Bashir H. Fetomaternal outcome in mild to moderate mitral stenosis. Pakistan Journal of Medical and Health Sciences. 2016;10(4):1348-1352.
- Fathy FT, Gonied AS, Mohammed NS. Maternal and neonatal outcome in women with cardiac diseases and suggested nursing guidelines. Journal of Nursing and Health Science. 2018;7(2): 80-91. Google Scholar
- Wasim T, Amer W, Majrroh A, Siddiq S. Foetomaternal outcome of pregnancy with cardiac disease. Journal-Pakistan Medical Association. 2008;58(4):175. PubMed | Google Scholar
- van Hagen IM, Thorne SA, Taha N, Youssef G, Elnagar A, Gabriel H. Pregnancy outcomes in women with rheumatic mitral valve disease. Circulation. 2018;137(8):806-816. PubMed | Google Scholar
- Sen M, Bhattacharyya P, Chowdhury N. Pregnancy with heart disease--fetomaternal outcome. Journal of Evolution of Medical and Dental Sciences. 2014;3(5):1178-1184. Google Scholar
- Anthony J, Osman A, Sani MU. Valvular heart disease in pregnancy. Cardiovascular journal of Africa. 2016;27(2):111-118. PubMed | Google Scholar
- Wolfe DS, Hameed AB, Taub CC, Zaidi AN, Bortnick AE. Addressing maternal mortality: the pregnant cardiac patient. American Journal of Obstetrics and Gynecology. 2019;220(2):167e1-167e8. PubMed | Google Scholar
- Diao M, Kane A, Ndiaye MB, Mbaye A, Bodian M, Dia MM et al. Pregnancy in women with heart disease in sub-Saharan Africa. Archives of Cardiovascular Diseases. 2011;104(6-7):370-374. PubMed | Google Scholar