Dolutegravir hypersensitivity reaction: the need for strengthening pharmacovigilance systems with optimization of antiretroviral therapy in HIV programs in resource-limited settings (case report)
Mukhtar Abdulmajid Adeiza, Isaac Kekulah, Ian Wachekwa, Onyema Ogbuagu
Corresponding author: Mukhtar Abdulmajid Adeiza, Department of Medicine, Ahmadu Bello University Teaching Hospital, PMB 06 Shika, Zaria, Nigeria 
Received: 21 Feb 2022 - Accepted: 11 Mar 2022 - Published: 18 Mar 2022
Domain: Infectious diseases epidemiology, Health system development, Infectious disease
Keywords: HIV, dolutegravir, adverse drug reaction, pharmacovigilance, resource-limited setting, case report
©Mukhtar Abdulmajid Adeiza et al. PAMJ Clinical Medicine (ISSN: 2707-2797). This is an Open Access article distributed under the terms of the Creative Commons Attribution International 4.0 License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cite this article: Mukhtar Abdulmajid Adeiza et al. Dolutegravir hypersensitivity reaction: the need for strengthening pharmacovigilance systems with optimization of antiretroviral therapy in HIV programs in resource-limited settings (case report). PAMJ Clinical Medicine. 2022;8:47. [doi: 10.11604/pamj-cm.2022.8.47.33930]
Available online at: https://www.clinical-medicine.panafrican-med-journal.com//content/article/8/47/full
Case report 
Dolutegravir hypersensitivity reaction: the need for strengthening pharmacovigilance systems with optimization of antiretroviral therapy in HIV programs in resource-limited settings (case report)
Dolutegravir hypersensitivity reaction: the need for strengthening pharmacovigilance systems with optimization of antiretroviral therapy in HIV programs in resource-limited settings (case report)
![]() Mukhtar Abdulmajid Adeiza1,2,3,&, Isaac Kekulah2,
Mukhtar Abdulmajid Adeiza1,2,3,&, Isaac Kekulah2, ![]() Ian Wachekwa2, Onyema Ogbuagu3,4
Ian Wachekwa2, Onyema Ogbuagu3,4
&Corresponding author
The incidence of antiretroviral therapy-related adverse drug reaction among people living with HIV in Liberia is unknown. For PLWH, unexplained new symptoms or deaths are often attributed to advanced HIV disease itself or opportunistic infections like tuberculosis. Surveillance systems for monitoring newly introduced combination antiretroviral drug treatments within HIV programs are either suboptimal or non-existent. Furthermore, when adverse drug reactions occur or are suspected by clinicians, the laboratory capacity for monitoring, diagnosis and medical treatment may be lacking. A 44-year woman diagnosed with HIV infection 5 years previously and on a regimen of lamivudine/zidovudine (3TC/AZT) and nevirapine (NVP) with viral suppression developed fever, rash and facial swelling 2 days after being transitioned to dolutegravir (DTG) based combination antiretroviral therapy and a backbone of lamivudine/tenofovir (3TC/TDF) as part of the Liberia National strategy for antiretroviral therapy treatment optimization. She was found to be acutely ill looking with fever, hives and angioedema but no features of anaphylaxis. Investigation was limited because of absence of routine laboratory monitoring, but her blood work showed eosinophilia. Following evaluation, a diagnosis of dolutegravir induced hypersensitivity reaction with angioedema was made. She was managed as outpatients on antihistamines and steroids. Dolutegravir (DTG) was also discontinued and substituted with ritonavir-boosted lopinavir (LPV/r) while retaining the 3TC/TDF backbone and relevant health education was provided. Symptoms resolved completely, and she was doing well at follow up 4 weeks later. We present a first case of hypersensitivity reaction leading to drug discontinuity following roll-out of dolutegravir as a first line cART in Liberia to call to attention the challenges of detecting and managing ART adverse drug reactions in a resource-limited setting and review the relevant literature to highlight the need for strengthening pharmacovigilance and laboratory monitoring systems in HIV programs in developing countries through planning, health systems strengthening and collaboration.
The increased utilization of combination antiretroviral therapy (cART) by people living with HIV (PLWH) has transformed HIV infection into a chronic but manageable disease and even though cART may cause short-term and long-term adverse effects, the benefit of cART far outweighs these events [1]. To achieve the UNAIDS 90/90/90 and 95/95/95 treatment targets for HIV/AIDS [2], optimization of current cART regimens is a critical strategy for National AIDS control programs [3,4]. By late 2019 123 countries including 41 low- and middle-income countries (LMICs) had included dolutegravir (DTG) containing regimens in their national protocols, as the preferred first-line option, particularly the fixed dose combination (FDC) of tenofovir disoproxil fumarate (TDF), lamivudine (3TC), and dolutegravir (DTG) [5]. The efficacy of this drug had been demonstrated in several randomized control trials (RCTs) conducted in both cART naïve and experienced patients, where it was superior to EFV based regimens [6-9]. In Liberia, following months of critical planning, transition to DTG based cART commenced in September 2019 to replace EFV based regimens. In general, the integrase strand transfer inhibitor (INSTI) class of cART are highly effective, well tolerated and associated with minimal drug interactions. In Randomized Controlled Trials (RCTs), the most frequent reported adverse effects were headache, nausea, diarrhoea, insomnia, and fatigue, but they were not severe enough to warrant discontinuing therapy [1]. In addition, many studies have concluded that INSTIs, particularly dolutegravir, lead to greater weight gain than other classes of antiretroviral, but the mechanism and clinical significance of this observation is unclear [10-11]. Rarely, mood changes, exacerbation or new onset of psychiatric disorders have also been observed [12]. Hypersensitivity reactions and hepatotoxity are thought to be rare [3,13]. Ideally, all persons with HIV who initiate cART should have laboratory studies performed at the initial visit, before initiating or changing a regimen, and regular monitoring for long-term safety, but the 2016 WHO HIV guidelines recommend a symptom-directed approach as opposed to laboratory monitoring of the safety and toxicity of cART regimens in resource limited settings [14]. The challenges with this recommendation include: a rise in adverse drug reactions (ADRs) with introduction of new antiretroviral drugs to optimize therapy for PLWH; unique challenges posed to clinicians in recognition, diagnosis and treatment of ADRs related to ARVs that may involve switching to available alternative regimens; absence of routine ART pharmacovigilance and laboratory monitoring which are not always built into HIV programs in sub-Saharan Africa and lack of funding and absence of ADR events surveillance systems that limit the ability to detect and characterize cART toxicity patterns. We present a case of a DTG-related hypersensitivity reaction leading to discontinuation of the drug with a review of the current literature, to highlight the need for heightened pharmacovigilance in HIV programs when new regimens are introduced in resource-limited settings.
Patient information: a 44-year-old woman who had been diagnosed with HIV 5 years earlier and on a first line regimen of lamivudine/zidovudine (3TC/AZT) and nevirapine (NVP) with full viral suppression presented to the infectious diseases clinic at John F. Kennedy Medical Center, Monrovia, Liberia in September 2019. She had no complaints and after being assessed and deemed eligible, she was transitioned to a new cART regimen of lamivudine/tenofovir (3TC/TDF) and dolutegravir (DTG) consistent with the latest recommendation by WHO and as adopted by the Liberian National AIDS and STI control Program (NACP). Two days after initiating the new regimen, she developed a rash and on the third day, noticed facial swelling. The rash was of sudden onset, diffuse, itchy and raised and associated with reddening of the skin with no characteristic pattern of progression but with associated photosensitivity on exposure to sunlight. She also developed itchy and red eyes but there were no oral or genital ulcers or and no hair or nail changes. A day after onset of the rash, she noticed hive-like swellings on her face, eyelids and lips that were more pronounced on the left side of the face but didn´t notice any tongue swelling. She reported fevers, fatigue and there was associated nasal congestion but no shortness of breath, sore throat, chest tightness, wheezing, stridor or cough. A review of other systems was unremarkable. She had a past medical history of seasonal allergies/atopy since childhood but on this occasion, there was no exposure to unusual foods, insect bites, other drugs, or any other possible triggers. She reported having elevated blood pressure in a past pregnancy that resolved after delivery. There was no history of alcohol ingestion or smoking and no family history of similar illness or bronchial asthma. She started cART in 2014 and had been on 3TC/AZT, NVP and cotrimoxazole which were well tolerated and there was no previous cART substitution, switch or treatment interruptions. She was single with 2 children who were well.
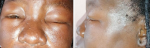
Clinical findings: on physical examination, she was found to be acutely ill looking and anxious but in no obvious respiratory distress. She was febrile with an axillary temperature of 37.6°C, a heart rate of 78 beats per minute, respiratory rate of 20 cycles/min and blood pressure of 129/82 mmHg. Her weight was 75kg, height was 1.58m and body mass index was 30.1kg/m2. There was no conjunctival pallor or cyanosis but she had red, injected conjunctivae with shotty submandibular lymphadenopathy. She had asymmetrical facial swelling with grossly edematous left eye and lips (Figure 1). There was no tongue swelling or protrusion and she had a generalized erythematous maculopapular rash with wheals and flare. She also had generalized facial and scalp tenderness but there were no nail or hair changes, her oral and genital mucosae were uninvolved. Otherwise, she had normal first and second heart sounds on cardiac exam, normal vesicular breath sounds on auscultation and was fully conscious and had no meningeal signs or focal neurological deficits.
Timeline of current episode: following diagnosis of HIV in 2014, patients was placed on appropriate first line regimen of 3TC/AZT/NVP until September 2019 when she was transitioned to 3TC/TDF/DTG as part of the new National ART strategy. She subsequently developed rash, facial swelling, fever and systemic symptoms within 2 days of the new regimen for which she presented for reevaluation.
Diagnostic assessment: her laboratory investigations revealed a hemoglobin of 11.7g/dl and a white blood cell count (WBC) of 6.8 x 103/μL. WBC differential showed a high eosinophil count of 10.5% (absolute count -714 cells/μL), lymphocytes -45%, neutrophils -36%, and monocytes -8.6%. The platelet count was 435 x 103/μL. Urinalysis was unremarkable and malaria smear was negative. Blood glucose level was 150mg/dl and test of liver function were unavailable due to financial limitations. Her CD4 T-lymphocyte count was 248 cells/μL and HIV RNA viral load was undetectable. There were no facilities for prick testing, patch testing and drug provocation testing.
Diagnosis: an impression of severe DTG induced hypersensitivity reaction with angioedema was made. Differential diagnosis was cutaneous ADR to cotrimoxazole but this was less likely because she had been exposed to this medication for 5 years with no previous reactions.
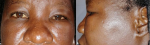
Therapeutic interventions: DTG was immediately discontinued and substituted with ritonavir-boosted lopinavir (LPV/r) while retaining the 3TC/TDF backbone. She was treated with a single dose of IV hydrocortisone 200mg followed by a 5-day course of oral prednisolone 30mg/day and chlorpheniramine 4mg at night. Following diagnosis and institution of appropriate therapy, our patient began to improve (Figure 2). Monitoring over the first few days was clinical and via phone calls with no worsening of symptoms or development of alarm signs. She was reviewed in the clinic on days 3 and 5 and the improvement was remarkable. Relevant education on the problem was also provided and symptoms resolved completely at 4 weeks follow-up and she is currently tolerating the new ARV regimen well with good viral load suppression.
Follow-up and outcome of interventions: following diagnosis and institution of appropriate therapy, our patient began to improve. Monitoring over the first few days was clinical and via phone calls with no worsening of symptoms or development of alarm signs. She was reviewed in the clinic on days 3 and 5 and the improvement was remarkable. Symptoms resolved completely at 4 weeks follow-up and she is currently tolerating the new ARV regimen well with good viral load suppression.
Patient perspective: I have been on antiretroviral drugs for 5 years and I was doing well on my previous regimen when it was suggested by the doctors during one of my visits that my drugs be changed because of the transition to the new DTG regimen. However, within days, I developed swelling on my face, skin rashes and started feeling very sick. Immediately I thought this could be due to the new drugs and I rushed back to the clinic. Though I couldn´t afford to do all the laboratory investigations out of pocket, I am happy that the doctors were able to recognize the problem and put me on appropriate medications and change my antiretroviral drugs to a regimen that is good and safe for me. I am happy with my recovery and I hope that in future, this program will have some support for investigating and treating patients who develop side effects to antiretroviral drugs.
Informed consent: for this case report, written informed consent was obtained from the patient to use the information and images.
Expected rise in antiretroviral therapy adverse drug reactions with new regimens and treatment optimization: antiretroviral therapy-related ADRs are common and are usually classified as mild, moderate, severe or life threatening [4]. With the prevailing test and treat strategy [14] and optimization of cART which results in initiating more patients on newer drugs, for which there is limited real world safety data from populations in resource-limited settings [15], an associated increase in ART adverse events is expected [1,3]. Generic fixed dose combination antiretroviral drug products are made available to developing countries through sponsorship of HIV/AIDS programs by The Global Fund to Fight AIDS, Tuberculosis and Malaria (GFATM) after WHO prequalification and the President's Emergency Program for AIDS Relief (PEPFAR) [3,16]. This is the first reported case of severe hypersensitivity reaction to DTG in Liberia and the patient was brought to attention only because of her return to the clinic after developing the unusual symptoms. There were no systems in place following rollout to detect ADRs to the new drug. After drug distribution, adequate national or regional quality and safety monitoring systems are lacking in resource-limited settings and ADRs tend to be under-recognized and underreported but this could be corrected by establishing and implementing an active surveillance process to proactively detect them [16].
Clinical management of antiretroviral therapy-related adverse drug reactions: in phase III clinical trials, DTG ADRs were reported in 1 to 4% of study participants, but were usually mild [6,7]. Hypersensitivity reactions leading to drug discontinuation were seen in less than 1% of study participants and were characterized by rash, constitutional symptoms, and occasionally organ dysfunction, including liver injury [3,13]. Hypersensitivity to DTG tends to begin early after commencing therapy, as was seen in the current case [1]. Although treat-through, rechallenge and desensitizing strategies for cART-related ADRs among PLWH may occur with success [4], the WHO recommends that DTG be discontinued and should not be reintroduced when severe life-threatening ADRs like severe rash or rash accompanied by fever, general malaise, fatigue, muscle or joint aches, blisters or peeling of the skin, oral blisters or lesions, conjunctivitis, facial edema, hepatitis, eosinophilia, angioedema, difficulty breathing occur [14]. The index patient had most of these severe symptoms, necessitating discontinuation of DTG therapy and switching to LPV/r [14]. Challenges may however arise where an acceptable substitute is not available, in such situations, the option of treating through i.e. rechallenge and desensitization may be considered especially in cases with less severe drug reactions as is done with antituberculosis drug reaction [4]. However, availability of desensitization protocols and emergency medications to manage severe reactions when they occur are limiting factors. Where there are competing potential differentials for hypersensitivity reaction including other drugs such as cotrimoxazole, capacity and expertise for skin prick or patch testing to adjudicate the accurate etiology is also lacking.
Monitoring of PLWH on antiretroviral therapy and building pharmacovigilance systems: with limited resources and managing complex systems, routine monitoring of cART has always been a challenge in sub-Saharan Africa. Some laboratory tests like CD4 counts and viral loads which are accepted monitoring standards are prioritized and funded [17] while routine laboratory tests such as complete blood count, chemistry panel and radiologic tests are frequently lacking and where available, costs are borne out of pocket by PLWH. For our patient, this gap in monitoring was obvious, as not all relevant investigations could be done due to challenges with out-of-pocket payment. However, the eosinophilia of 10.5% (714 cells/μL) was highly suggestive of ADR. This laboratory monitoring gap becomes even more acute when it comes to event-triggered monitoring needs like cART ADR. In the absence of laboratory systems for this purpose, the 2016 treatment guideline by the WHO recommends that National HIV programs adopt a symptom-directed approach. This event triggered process at best helps to identify and address individual patient needs, but most treatment sites are not able to organize and use findings from these random events for proper decision-making and planning at a programmatic level [14]. It is suggested that a properly organized routine surveillance process that pools and analyses data generated from such identified cases through a systematic HIV program directed pharmacovigilance system will help define the pattern of ART ADR in resource limited settings and highlight the peculiarities with respect to the interaction of ART with food and nutrition, coinfections like tuberculosis and their treatments, use of alternative medicines and other risk factors for ADRs [18]. The potential benefits of proactive ADR monitoring will include PLWH counselling and education, prompt identification and characterization of ADRs when newer drugs are introduced in new populations, prevention of adherence problems and subsequent treatment failure that can result from ADRs, averting unnecessary blind ARV substitution and switching, ensuring patient safety, and prioritizing ADR funding needs.
Prioritization, collaboration, funding and post roll-out surveillance: few resource-limited countries have all the structures, systems, or funding resources necessary to support medicines safety activities [19]. This is also true for HIV programs and may be related to donor or partner funding priorities or failure of national programs to identify this critical area of need. To overcome this, systems must be put in place within national programs to build a locally implementable pharmacovigilance system for ARVs that brings together the competing and sometimes contrasting priorities that exist in implementing HIV programs, namely: the need for delivery of care and treatment services; an active as opposed to a passive ADR surveillance systems; pharmaceutical industry support; country ownership; international collaboration; research priorities; and programmatic funding [20]. At present, such systems are not in place in Liberia and most ADRs are probably missed or undocumented. Where ADRs are recognized, physicians face challenges of properly investigating, monitoring and characterizing the event as most patients are unable to afford out-of-pocket payment for laboratory services and drug treatments that are not covered by HIV programs. This can make the distinction between ADRs, new opportunistic infections and HIV treatment failure difficult and challenging in HIV clinical service delivery.
Following implementation of changes in treatment guidelines, the toxicity profiles of INSTIs will also need further exploration through post roll-out surveillance of ARVs in resource-limited settings, and identify risk factors for these severe reactions [14]. As some ADRs occur in the long-term, this would need to be sustained over a long period of time. A laboratory-driven monitoring approach, distinct from a symptom directed approach, is more likely to lead to earlier identification of ADRs, but it remains to be seen if this more resource-intensive approach will be supported by National programs, international partners or funders through collaborative activities considering the low socio-economic status of PLHIV in resource-limited settings. In this case our patient was only able to do some of her investigations and until the time of symptom resolution, liver function tests were still not available, but her high eosinophil count was highly suggestive of an ADR. It is suggested that since most PLWH are periodically attending clinics, there is an opportunity for prevention, routine monitoring and early intervention and for ADRs reflected either as clinical symptoms or silent laboratory abnormalities [1]. However, it is essential for National programs and partners to integrate these pharmacovigilance structures within ART services to enhance early detection of ADRs in developing countries, where resources are not readily available for comprehensive monitoring of ART ADRs.
Primary take away points in this case report include: (i) following roll-out of new antiretroviral drugs for optimization of antiretroviral therapy in resource-limited settings, there is a need to institute pharmacovigilance systems to monitor ART-related ADR; (ii) severe hypersensitivity reactions to INSTIs develop early after commencing therapy and PLWH education, a high index of suspicion and close monitoring by clinicians is important. Data gathered from identified cases of antiretroviral drug ADR should be pooled and analysed by National HIV programs to determine local ADR patterns to inform decision-making, planning and prevention; (iii) prioritization of antiretroviral therapy laboratory monitoring activities by National programs through surveillance, improved laboratory infrastructure and funding in collaboration with partners and donors is essential to early identification of subclinical and clinical ADRs to prevent fatal outcomes.
The authors declare no competing interests.
MAA was responsible for conception, design of the report, editing and approval of the final draft; IK contributed to design of the report, editing and approval of the final draft; IW contributed to design of the report, editing and approval of the final draft; OO contributed to the report and was responsible for editing and approval of the final draft. All the authors have read and agreed to the final manuscript.
The authors thank the staff at the Infectious disease clinic and the Department of Medicine at the John F Kennedy Medical Center, Monrovia, Liberia for their support.
Figure 1: A,B) front and side view of facial and eyelid edema at presentation
Figure 2: A,B) front and side view of resolving facial and eyelid edema on day 5
- Fernandez-Montero JV, Eugenia E, Barreiro P, Labarga P, Soriano V. Antiretroviral drug-related toxicities-clinical spectrum, prevention, and management. Expert Opinion on Drug Safety. 2013 Sep;12(5):697-707. PubMed | Google Scholar
- UNAIDS. Understanding fast-track - accelerating action to end the AIDS epidemic by 2030. 2015. Accessed July 31, 2020.
- World Health Organization. Dolutegravir (DTG) and the fixed dose combination (FDC) of tenofovir/lamivudine/dolutegravir (TLD) briefing note 2018. Accessed April 21, 2020.
- Peter J, Choshi P, Lehloenya RJ. Drug hypersensitivity in HIV infection. Curr Opin Allergy Clin Immunol. 2019;19(4):272-282. PubMed | Google Scholar
- Aidsmap. Dolutegravir recommended for all in new World Health Organization guidelines. 2019. Accessed July 31, 2020.
- Walmsley SL, Antela A, Clumeck N, Duiculescu D, Eberhard A, Gutiérrez F et al. Dolutegravir plus abacavir-lamivudine for the treatment of HIV-1 infection. N Engl J Med. 2013 Nov 7;369(19):1807-18. PubMed | Google Scholar
- Stellbrink HJ, Reynes J, Lazzarin A, Voronin E, Pulido F, Felizarta F et al. Dolutegravir in antiretroviral-naïve adults with HIV-1: 96-week results from a randomized dose-ranging study. AIDS. 2013 Jul 17;27(11):1771-8. PubMed | Google Scholar
- Clotet B, Feinberg J, van Lunzen J, Khuong-Josses MA, Antinori A, Dumitru I et al. Once daily dolutegravir versus darunavir plus ritonavir in antiretroviral-naive adults with HIV-1 infection (FLAMINGO): 48 week results from the randomised open-label phase IIIb study. Lancet. 2014;383(9936):2222-31. PubMed | Google Scholar
- Trottier B, Lake JE, Logue K, Brinson C, Santiago L, Brennan C et al. Dolutegravir/abacavir/lamivudine versus current ART in virally suppressed patients (STRIIVING): a 48-week, randomized, non-inferiority, open-label, Phase IIIb study. Antivir Ther. 2017;22(4):295-305. PubMed | Google Scholar
- Galdamez R, García JA, Fernández M, Robledano C, Agulló V, García-Abellán J et al. Short-term Increase in risk of overweight and concomitant systolic blood pressure elevation in treatment-naïve persons starting INSTI-based antiretroviral therapy. Open Forum Infect Dis. 2019;6(12):ofz491. PubMed | Google Scholar
- Bourgi K, Rebeiro PF, Turner M, Castilho JL, Hulgan T, Raffanti SP et al. Greater weight gain in treatment-naive persons starting dolutegravir-based antiretroviral therapy. Clin Infect Dis. 2020;70(7):1267-1274. PubMed | Google Scholar
- Kheloufi F, Allemand J, Mokhtari S, Default A. Psychiatric disorders after starting dolutegravir: report of four cases. AIDS. 2015;29(13):1723-1725. PubMed | Google Scholar
- Rivero A, Domingo P. Safety profile of dolutegravir. Enferm Infecc Microbiol Clin. 2015;33 Suppl 1:9-13. PubMed | Google Scholar
- World Health Organization. Geneva. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach - Second edition 2016. Geneva. Accessded April 2, 2020.
- Teklay G, Legesse B, Legesse M. Adverse effects and regimen switch among patients on antiretroviral treatment in a resource limited setting in Ethiopia. J Pharmacovigilance 2013;1:115. Google Scholar
- Miller V, Nwokike J, Stergachis A. Pharmacovigilance and global HIV/AIDS. Curr Opin HIV AIDS. 2012;7(4):299-304. PubMed | Google Scholar
- Klimarx PH, Simbi R. Progress and challenge in scaling up laboratory monitoring of HIV treatment. Plos Med. 2016;13(8):e1002089. PubMed | Google Scholar
- World Health Organization. Pharmacovigilance for antiretrovirals in resource-poor countries. Geneva 2007. Accessed May 25, 2020.
- Olsson S, Pal SN, Stergachis A, Couper M. Pharmacovigilance activities in 55 low- and middle-income countries: a questionnaire-based analysis. Drug Saf. 2010;33(8):689-703. PubMed | Google Scholar
- Bakare N, Edwards IR, Stergachis A, Pal S, Holmes CB, Lindquist M et al. Global pharmacovigilance for antiretroviral drugs: overcoming contrasting priorities. PLoS Med. 2011;8(7):e1001054. PubMed | Google Scholar