Management modalities of pulmonary hypertension post COVID-19: about a monocentric observational study of 28 cases
Nassime Zaoui, Amina Boukabous, Nadhir Bachir, Ali Terki, Meriem Bougdour
Corresponding author: Nassime Zaoui, Cardiology Department, Omar Yacef Draa Ben Khedda Hospital, Tizi-Ouzou Medical University, Tizi-Ouzou, Algeria 
Received: 20 Apr 2022 - Accepted: 09 Jun 2022 - Published: 16 Jun 2022
Domain: Cardiology, Infectious disease, Intensive care medicine
Keywords: Series, COVID-19, SARS-CoV-2, pulmonary hypertension, precapillary, Bosentan, prostaglandin
©Nassime Zaoui et al. PAMJ Clinical Medicine (ISSN: 2707-2797). This is an Open Access article distributed under the terms of the Creative Commons Attribution International 4.0 License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cite this article: Nassime Zaoui et al. Management modalities of pulmonary hypertension post COVID-19: about a monocentric observational study of 28 cases. PAMJ Clinical Medicine. 2022;9:12. [doi: 10.11604/pamj-cm.2022.9.12.35028]
Available online at: https://www.clinical-medicine.panafrican-med-journal.com//content/article/9/12/full
Case series 
Management modalities of pulmonary hypertension post COVID-19: about a monocentric observational study of 28 cases
Management modalities of pulmonary hypertension post COVID-19: about a monocentric observational study of 28 cases
Nassime Zaoui1,&, Amina Boukabous1, Nadhir Bachir1, Ali Terki1, Meriem Bougdour1
&Corresponding author
Pulmonary hypertension (PH) is a rare pathology recognizing five groups of different mechanisms and etiologies. Its diagnosis is suspected by the clinical examination and ECG and confirmed by echocardiography and cardiac catheterism; its treatment is based on general rules, first and second line treatment (diuretics, oxygenotherapy, anticoagulants and calcium-channel blockers) and specific therapies for group 1 in case of negative invasive reversibility test (ET1 antagonist, phosphodiesterase inhibitors, prostaglandin analogues). Since the beginning of COVID-19 pandemic, post-mortem works suggest the emergence of a new form of PH related to pulmonary arterial walls thickening, confirmed by clinical observations. Many grey areas persist regarding the management of this new entity. Objective: to describe therapeutic modalities in ambulatory patients with PH-post-COVID-19 and to compare echocardiographic evolution (PAP and right ventricle diameter) according to the chosen therapeutic regimen. This retrospective observational, single-center study conducted in 2021 involved 28 patients with PH-post-COVID-19 confirmed with echocardiography and cardiac catheterism. Patients with previous history of PH or evidence of pulmonary embolism were excluded (n=04). According to invasive reversibility test, patients were divided in two groups, with different treatment regimen (Calcium channel blockers VS Bosentan+Iloprost) and followed for 3 months. Transthoracic echocardiography (TTE) findings were compared in these two groups at the first and third treatment month. Twenty-eight patients (17 men and 11 women from 26-64 years) with post-COVID-19 PH (severe COVID-19 form: lung involvement >50% at the initial CT) and post-infection time of 28 ± 4 days, were included. All patients had positive ESR and CRP at baseline. Average sPAP in TTE was 80 ± 15 mmHg. Mean telediastolic RV diameter was 43 ± 7 mm/m2. Cardiac catheterization revealed a positive reversibility test in nine patients (put on Diltiazem) and negative in 19 patients (put on Bosentan+Iloprost). All patients reported functional improvement, without significant improvement in echocardiographic parameters at 1 month (Δ sPAP at 7 ± 3 mmHg and Δ RV Diameter 4 ± 2 mm) and 3 months follow-up (Δ sPAP 10 ± 2 mmHg and Δ RV Diameter 6 ± 2 mm). This improvement was greater in Bosentan + Iloprost group compared to Diltiazem group for sPAP and RV diameter at 3 months (Δ sPAP 13 ± 4 mmHg VS Δ sPAP 4 ± 2 mmHg, P at 0.07 and Δ RV 8 ± 3 mm VS Δ RV 3 ± 2 mm, P at 0.08). Severity of lung involvement, oxygen requiring and the persistence of biological inflammation appear to be associated with a more unfavorable course. Symptomatic evolution seems favorable under treatment however a longer delay is necessary to confirm an objective TTE improvement that seem higher with specific therapeutics, suggesting rapid worsening of vascular parietal rearrangement that may explain poorer results despite a positive reversibility test. PH-post-COVID-19 requires early identification and targeted management to improve patient prognosis. The specific treatment of PH could be the first intension treatment of this entity, regardless of reversibility test results.
Pulmonary hypertension (PH) is a rare and little-known pathology [1] defined, according to the Sixth World Congress of the PH (Nice 2018), as a mPAP ≥ 20 mmHg [2,3]; there is not enough data to define stress PH, the old definition that speaks about PH if mPAP ≥30 mmHg was dropped in 2008 due to a wide variability in normal pulmonary stress pressure value [2,3]. Its prevalence is estimated at 97 cases per million inhabitants with a ratio ♀/♂ of 1.8 (United Kingdom) [2,4]; its mortality is estimated at 4.5-12.3 per 10,000 inhabitants (USA) [2-4]. PH is classified into 5 groups according to etiology and pathophysiological mechanism (Table 1) [3,5]. The diagnosis is suspected by symptomatology and clinical examination [1-3], then, specified by simple explorations such as ECG [2] and chest X-ray and confirmed by transthoracic echocardiography (TTE) and cardiac catheterization [2,6]. Other investigations, such as lung function tests, arterial gasometry, ventilation/perfusion pulmonary scintigraphy, and chest CT scan look for lung or airway disease and chronic thrombo-embolic pulmonary hypertension (CTEPH) [4,6]. Cardiac MRI assesses the size, morphology and function of RV in poor quality TTE [6]. The management of PAH in group 1 is divided into 4 components [2,3]: general measures: pregnancies eviction, annual influenza and pneumococcal every 5 years vaccination, moderate and supervised physical activity is advised while intense physical activity is prohibited, in the event that a surgical procedure is planned, the epidural is preferable. First and second line treatment: diuretics, long-term oxygen therapy (16 to 18H/24H if oxygen blood pressure is < 60 mmHg, Objective > 65 mmHg), oral anticoagulation and high-dose calcium channel blockers in case of positive reversibility test in cardiac catheterization. Specific treatment: vasodilator, antiproliferative and antifibrotic effect with 3 molecules: prostacyclines and analogues (inhaled iloprost and treprostinil subcutaneous); endothelin antagonist ET-1 (bosentan); phosphodiesterase-5 inhibitors (sildenafil, tadalafil). Other treatments: Inotropes in case of hypotension, heart lung transplantation and septal balloon atriotomy. In group 2 and 3 PH, management is based on the specific treatment of causal pathology, the use of PH-specific therapies in these two groups is not indicated. In group 4 PH, lifelong anticoagulation and surgical endarterectomy are indicated, balloon pulmonary dilation may be considered if the risk of surgery is significant; the use of specific PH therapies (in mono or dual therapy) is possible.
Rationale: since the end of 2019, the world has been experiencing an unprecedented pandemic of COVID-19; data from the literature suggest a low incidence of viral involvement in patients with PH [7]. An international survey of reference centers for PH in 28 countries, confirmed this low incidence with however a higher mortality rate than in the general population (19% vs 3-5%) [8]. Pulmonary arterial vasoconstriction and hypoxemia, during PH, causes endothelial dysfunction and polycythemia, both responsible for an increased release of endothelial and red cells NO. NO excess could explain that endothelial dysfunction of the hyper inflammatory phase of COVID-19, with its thrombotic consequences, is less [9] and would play a role in viral replication, Indeed, Akerström et al. in 2005 proved that NO inhibits the coronavirus replication cycle in SARS-CoV-1 of H1N1, it could be identical in COVID-19 [8,9]. Post-mortem work has suggested, however, that this pandemic could lead to the emergence of a new form of PH by highlighting thickened pulmonary arterial walls in patients who died of COVID-19 [10], these histological lesions were not found in SARS-CoV-1 of H1N1 influenza in 2002-2004 [10,11]. Clinical observations confirmed this hypothesis [11,12]. Many series suggest the use of NO in the most severe forms or in the acute phase, with a clear proportion of functional improvement [13]. Many grey areas persist regarding the management of this new entity. The long-term treatment, and in the absence of evidence, currently uses the same traditional regimen as for the rest of group 1 PAH with general measures, first-line treatments, calcium channel blockers in reversible forms and specific therapies in irreversible forms with, in first-line, dual therapy [14]. Most of the series described in the literature report a poorer prognosis of this new form compared to other cases of non-COVID-19-PAH despite well-conducted therapy [14].
Objective: the objective of this study is to describe therapeutic and evolutionary modalities of patients with post-COVID-19 PH and to compare the evolution of the PH echocardiographic parameters (PAP and right ventricle diameter) according to the chosen therapeutic regimen (calcium channel blockers or specific treatment in dual therapy: Bosentan + Iloprost).
Study design: this is an observational, retrospective, single-center study.
Setting: the study was conducted from October 2021 to April 2022, during the COVID-19 pandemic, in a PH management center, from a prospective registry collecting clinical, biological and imaging data on PH patients (from all forms). Patients who were registered in our PH registry from October 2021 to January 2022 and met the inclusion criterion for this work were enrolled in the study. A follow-up period of 3 months was observed in all these patients. Data collection lasted until April 2022.
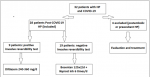
Participants: were included in the study, all patients referred to our center for post-COVID-19 PH and confirmed in TTE and cardiac catheterism (total of 32 patients). Were excluded from the study, patients with previous history of PH or evidence of pulmonary embolism (n=04). According to the invasive reversibility test, the patients were divided into two groups, with a different treatment regimen (Diltiazem high dose VS Bosentan + Iloprost) given by hospital according to the usual protocol of management of PH in our center, and followed for 3 months. Functional status and echocardiographic TTE findings were compared in these two groups at first and third month of follow-up. All participants gave their informed consent to participate retrospectively in this study and share the results.
Variables: we collected for all patient's functional status and walking perimeter, echocardiogramphy and cardiac cathetersim.
Measurement: functional status and walking perimeter were evaluated by interrogation and mentioned in the patient's medical record and in the PH registry of the department at each consultation (first, 1 and 3 months). Echocardiographic parameters were measured on General Electic (GE) ultrasound machine at each consultation (first, 1 and 3 months). A summary of the report has been archived in the patient's medical record and in the department's PH registry The RV diameter (RVd) was measured in telediastal 4 cavities apical window and automatically indexed to the body surface [2,3]. Systolic PAP (sPAP) was estimated by tricuspid regurgitation flow with formula: SPAP=4xtricuspid regurgitation (TR) peak velocity2+right atrial pressure (RAP) [2,3]. Cardiac catheterism was performed on GE Optima machine in the first visit, the reversibility test was performed in all patients either by NO (20 ppm) or inhaled Iloprost (2.5μg) according to the operator's preferences, and was considered positive any test showing decrease of 10 mmHg in PAP (absolute value < 40 mmHg) without decrease in cardiac output [2]. A summary of the report has been archived in the patient's medical record and in the department's PH registry.
Biases: selection bias: In order to reduce these biases and make the study population as representative as possible of daily practice, we did not limit the origin of patients whose recruitment was successive, as a reminder, our center is a convergent center for PH patients.
Verification bias: all patients included in the study received the mandatory reference test (sPAP measurement at cardiac catheterism).
Interpretation bias: faced with the risk of contamination and the workload imposed by the pandemic, we did not perform a double-blind evaluation for the two tests in our study (TTE and cardiac catheterism) but each parameter was confirmed by the same operator on two different measurements.
Study size: we have included consecutively all patients with the inclusion criterion (PH post-COVID-19) from October 2021 to January 2022 bringing the total number of patients to 32 then after applying the exclusion criterion (previous history of PH or evidence of pulmonary embolism), 28 patients were retained in this work.
Quantitative variables: based on PAP response on reversibility test, we divided our patients in two groups: positive reversibility test (receiving Diltiazem) and negative reversibility test (receiving Bosentan + Iloprost).
Statistical methods: all data were collected on the EPI-INFO 7 software. Results were expressed as a percentage for qualitative variables and average ± standard deviation (SD) for quantitative variables. Bivariate analyses of TTE parameters evolution according to the therapeutic schema were carried out according to the χ2 and the Fisher test for qualitative variables and Student's test for quantitative variables. P-value <0.10 was considered statistically significant.
Ethics approval and consent to participate: the hospital's ethics committee has given its consent to carry out this study and share the results under the number 097/22. All participants gave their informed consent to participate retrospectively in this study and to share the results.
Participants: a total of 32 patients were included in our study and then after the analysis of the exclusion criterion 4 patients were excluded, bringing the final number of patients to 28 who all participated in the inclusion visit and the 1 and 3-month control visit.
Descriptive data: twenty-eight patients (17 men and 11 women) aged from 26 to 64 years (average age 47 ± 3.4 years) with post-COVID-19 PH confirmed in TTE and right cardiac catheterization were included (Figure 1). All patients had a severe form of COVID-19 (lung involvement >50% at the initial CT scan) and required hospitalization and high-flow oxygenation (> 10 l/min). Patients were referred to our department with a post-COVID-19 infection delay between 15-45 days, with an average of 28 ± 4 days. Half of the patients had a positive dimers level (mean value at 2300 μg/L) at the initial infection, this data was missing in eight patients while the other six patients had a normal level (<500 μg/L). All patients had ESR and CRP positive at baseline, showing persistent inflammation. The mean sPAP on echocardiography was 80 ± 15 mmHg. The mean RVd was 43 ± 7 mm/m2 with RV dilation in 21 patients (75%). Cardiac catheterization revealed PH in all our patients, with an average PAP value of 58 ± 8 mmHg. A reversibility test came back positive in 9 patients, allowing to put them on Diltiazem 240 to 360 mg divided into 3 times a day, and negative in 19 patients requiring a combination: Bosentan 125 mg x 2/day with Iloprost inhaled 6 times a day.
Outcome data: the therapy was initiated intra-hospital and then the patients were discharged according to their clinical course.
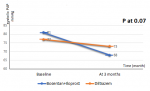
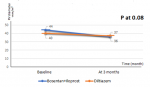
Main results: all patients (100%) were reviewed at 1 and 3 months after initiation of treatment and reported an improvement in functional status and walking perimeter without significant improvement in echocardiographic parameters at 1 month (Δ sPAP at 7 ± 3 mmHg and Δ RVd 4 ± 2 mm) and at 3 months (Δ sPAP 10 ± 2 mmHg and Δ RVd 6 ± 2 mm) (Table 2, Table 3). However, this improvement was higher in Bosentan + Iloprost group compared to Diltiazem group for echocardiographic sPAP and RVd at 3 months (Δ sPAP 13 ± 4 mmHg VS Δ sPAP 4 ± 2 mmHg, P 0.07 and Δ RVd 8 ± 3 mm VS Δ RVd 3 ± 2 mm, P 0.08) (Table 4, Figure 2, Figure 3).
Key results and interpretation: the symptomatic evolution seems favorable under treatment; however, a longer follow-up and monitoring time seems necessary to confirm an objective improvement in PAP and RVd that is more favorable with specific PH therapeutics compared to calcium channel blockers suggesting a persistent and rapid evolution of the vascular parietal rearrangement phenomenon that may explain less good echocardiographic results despite a positive reversibility test.
Limitations: the small number of patients in our series does not allow identifying predictive factors of installation of pulmonary hypertension after COVID-19 infection; however, it seems that the severity of lung involvement on CT as well as the use of oxygenation therapy and the persistence of biological inflammation after COVID-19 infection are associated with a more unfavorable course. The high level of dimers also seems to be associated with a move towards PH but the missing data in our series prevent any conclusion in this direction.
Interpretation: PH-post-COVID-19 requires early identification and targeted management to improve patient prognosis. The specific treatment of PH could be, in the future, the first line treatment of this entity, regardless of reversibility test results.
Generalisability: we remain cautious in the analysis of these results because the follow-up period and the number of patients are low and we call on other centers to converge their results to obtain greater statistical power.
PH-post-COVID-19 requires early identification and targeted management to improve patient prognosis. In the future, the specific treatment of PH could be the first line treatment of this entity, regardless of reversibility test results.
What is known about this topic
- Post-COVID-19 PH is a new entity increasingly described in the literature; it is part of group 1 and is due to a thickening of the pulmonary arteries walls;
- The treatment of this form uses drugs dedicated to the PH of the first group;
- The prognosis appears to be poorer than in the general population.
What this study adds
- The severity of lung involvement and the persistence of inflammation after the infectious phase seem to predispose to the onset of PH;
- Patients put on specific treatment have a better evolution than patients put on calcium channel blockers and this despite a positive reversibility test, leading to conclude that parietal thickening is persistent and rapidly scalable.
The authors declare no competing interests.
NZ was responsible for the design of the study, participated in the realization of cardiac catheterisms, interpreted the results and participated in the writing of the manuscript. AT participated in the realization of echocardiographies and carried out the analysis and statistical tests. AB participated in the analysis and interpretation of the results and the realization of echocardiographies. NB participated in the realization of cardiac catheterisms and in the writing of the manuscript. MB participated in the realization of the echocardiographies and in the writing of the manuscript. All the authors have read and agreed to the final manuscript.
We thank our paramedics who participated in the explorations carried out in this study and also our medical secretaries who ensured the archiving of the patients data.
Table 1: PH Classification according to the 6th World Symposium on Pulmonary Hypertension 2018
Table 2: improvement of echocardiographic parameters after 1 month in the global study population
Table 3: improvement of echocardiographic parameters after 3 months in the global study population
Table 4: comparison of improvement in echocardiographic parameters after 1 and 3 months between the 2 study groups
Figure 1: study flow diagram
Figure 2: sPAP improvement according to the PH treatment
Figure 3: RV diameter improvement according to the PH treatment
- Ryan JJ, Thenappan T, Luo N, Ha T, Patel AR, Rich S et al. The WHO classification of pulmonary hypertension: a case-based imaging compendium. Pulm Circ. 2012 Jan-Mar;2(1):107-21. PubMed | Google Scholar
- Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A et al. ESC Scientific Document Group. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2016 Jan 1;37(1):67-119. PubMed | Google Scholar
- Barberà JA, Román A, Gómez-Sánchez MÁ, Blanco I, Otero R, López-Reyes R et al. Guidelines on the diagnosis and treatment of pulmonary hypertension: summary of recommendations. Arch Bronconeumol (Engl Ed). 2018 Apr;54(4):205-215. PubMed | Google Scholar
- Hoeper MM, Humbert M, Souza R, Idrees M, Kawut SM, Sliwa-Hahnle K et al. A global view of pulmonary hypertension. Lancet Respir Med. 2016 Apr;4(4):306-22. PubMed | Google Scholar
- Simonneau G, Montani D, Celermajer DS, Denton CP, Gatzoulis MA, Krowka M et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019 Jan 24;53(1):1801913. PubMed | Google Scholar
- Mandras SA, Mehta HS, Vaidya A. Pulmonary hypertension: a brief guide for clinicians. Mayo Clin Proc. 2020 Sep;95(9):1978-1988. PubMed | Google Scholar
- Castiglione L, Droppa M. Pulmonary Hypertension and COVID-19. Hamostaseologie. 2021 Dec 21. Google Scholar
- Sulica R, Cefali F, Motschwiller C, Fenton R, Barroso A, Sterman D. COVID-19 in pulmonary artery hypertension (PAH) patients: observations from a large PAH Center in New York City. Diagnostics (Basel). 2021 Jan 15;11(1):128. PubMed | Google Scholar
- Horn E, Chakinala MM, Oudiz R, Joseloff E, Rosenzweig EB. Author rebuttal to response regarding letter to the editor regarding “could pulmonary arterial hypertension patients be at lower risk from severe COVID-19”. Pulm Circ. 2020 Jun 23;10(3). Google Scholar
- Suzuki YJ, Nikolaienko SI, Shults NV, Gychka SG. COVID-19 patients may become predisposed to pulmonary arterial hypertension. Med Hypotheses. 2021 Feb;147:110483. PubMed | Google Scholar
- Tudoran C, Tudoran M, Lazureanu VE, Marinescu AR, Pop GN, Pescariu AS et al. Evidence of pulmonary hypertension after SARS-CoV-2 infection in subjects without previous significant cardiovascular pathology. J Clin Med. 2021 Jan 7;10(2):199. PubMed | Google Scholar
- Khan A W, Ullah, I, Khan K S, Tahir M J, Masyeni S, Harapan, H. Pulmonary arterial hypertension post COVID-19: a sequala of SARS-CoV-2 infection. Respiratory Medicine Case Reports. 2021;33:101429. PubMed | Google Scholar
- Zamanian RT, Pollack CV Jr, Gentile MA, Rashid M, Fox JC, Mahaffey KW et al. Outpatient Inhaled nitric oxide in a patient with vasoreactive idiopathic pulmonary arterial hypertension and COVID-19 Infection. Am J Respir Crit Care Med. 2020 Jul 1;202(1):130-132. PubMed | Google Scholar
- Pagnesi M, Baldetti L, Beneduce A, Calvo F, Gramegna M, Pazzanese V et al. Pulmonary hypertension and right ventricular involvement in hospitalised patients with COVID-19. Heart. 2020 Sep;106(17):1324-1331. PubMed | Google Scholar