Gastric perforation in a 1-day-old newborn revealed by massive pneumoperitoneum: a case report
Koama Adjirata, Tiemtore-Kambou Bénilde Marie-Ange, Ouedraogo Somkieta Francis Modeste, Ciss Rabiou
Corresponding author: Koama Adjirata, Department of Medical Imaging And Interventional Radiology of The Teaching Hospital of Bogodogo, Bogodogo, Burkina Faso 
Received: 24 Mar 2023 - Accepted: 08 May 2023 - Published: 25 May 2023
Domain: Neonatology
Keywords: Gastric perforation, newborn, pneumoperitoneum, case report
©Koama Adjirata et al. PAMJ Clinical Medicine (ISSN: 2707-2797). This is an Open Access article distributed under the terms of the Creative Commons Attribution International 4.0 License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cite this article: Koama Adjirata et al. Gastric perforation in a 1-day-old newborn revealed by massive pneumoperitoneum: a case report. PAMJ Clinical Medicine. 2023;12:7. [doi: 10.11604/pamj-cm.2023.12.7.39791]
Available online at: https://www.clinical-medicine.panafrican-med-journal.com//content/article/12/7/full
Case report 
Gastric perforation in a 1-day-old newborn revealed by massive pneumoperitoneum: a case report
Gastric perforation in a 1-day-old newborn revealed by massive pneumoperitoneum: a case report
![]() Koama Adjirata1,&, Tiemtore-Kambou Bénilde Marie-Ange1,2,
Koama Adjirata1,&, Tiemtore-Kambou Bénilde Marie-Ange1,2, ![]() Ouedraogo Somkieta Francis Modeste3, Ciss Rabiou2,4
Ouedraogo Somkieta Francis Modeste3, Ciss Rabiou2,4
&Corresponding author
Gastric perforation in newborns is a rare but serious pathology. We report the case of a 1-day-old newborn in order to describe our diagnostic approach, our therapeutic conduct in a context of limited resources, and also to discuss the complexity of establishing an etiological diagnosis. This was a 1-day-old newborn, born at term weighing 2800 grams, who presented with respiratory distress within the hour following birth, then abdominal distension within 24 hours, followed by fever. Plain abdominal X-ray performed on the first day of life showed massive pneumoperitoneum. Surgical exploration showed a gastric perforation of 1.5 cm, localised on the greater curvature of the stomach. The evolution was marked by death on three days postoperative. Gastric perforation in newborns is a medico-surgical emergency. His prognosis remains grim. It could be improved by diligent care.
Gastric perforation in newborns is a rare but serious pathology due to its high mortality [1-3]. Two entities are described in relation to etiology. These are spontaneous or idiopathic gastric perforation, most often due to unidentified underlying diseases and traumatic or iatrogenic gastric perforation following attempts to insert a nasogastric tube [1,2,4]. Whether idiopathic or traumatic, neonatal gastric perforation is a medico-surgical emergency requiring rapid diagnosis and diligent management [1,4]. There are few series reported in the literature. Most of the writings on the subject are case reports, including three from Black Africa [3,5,6]. The purpose of this work was to report the first Burkinabé case in the literature and the 4th from Black Africa, in order to describe our diagnostic approach and our therapeutic conduct in a context of limited resources.
Patient information: this was a male newborn on first of life who presented with respiratory distress, abdominal distension, fever and absence of meconium emission. He is the 4th of a uterine sibling of 04 children, of which the 03 others were all alive, in good apparent health with no particular pathological background. He was born of a full-term pregnancy (38 weeks of amenorrhea), regularly followed up with three prenatal consultations, antimalarial prophylaxis based on sulfadoxine-pyrimetamine, antianemic prophylaxis based on iron + folic acid and three doses of vaccine tetanus. The pregnancy proceeded without any particular incident. The delivery was vaginal in the maternity hospital of the Teaching Hospital of Bogodogo, of a newborn of 2800 grams with an Apgar score of 7; 7 and 9 respectively at 00 minute, 05 minutes and 10 minutes. Within an hour of birth, he presented with respiratory distress and amniotic fluid aspiration was suspected. The newborn was immediately transferred to the neonatology department of the Bogodogo Teaching Hospital. He benefited from a nasopharyngeal aspiration, the establishment of a nasogastric tube and the taking of a venous line with the start of treatment based on antibiotics and oxygen at 2 liters/min.
Clinical findings: it was persistence of respiratory distress, the appearance of abdominal distension, fever and the absence of meconium emission. Hirchprung's disease was suspected in the first time. Clinical examination revealed good general condition, good state of consciousness, good mucocutaneous coloration, archaic reflexes were present. The temperature was 38.7°C, the heart rate 130 beats/min. Respiratory rate was 52 cycles/min and oxygen saturation were 96% on oxygen at 2 liters/min. There was abdominal distension with collateral venous circulation, difficult to palpate, tympanism on percussion and an absence of air-fluid noise on auscultation. Rectal examination was not done.
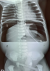
Diagnosis assessment: radiograph of the abdomen without preparation (RASP) was requested. The RASP performed at the radiology department of the Bogodogo Teaching Hospital on the night of August 8, 2021 to August 22, 2022 showed significant pneumoperitoneum pushing the stomach back into the left flank with the presence of a nasogastric tube in place and free fluid effusion into the peritoneal cavity (Figure 1). The diagnosis of peritonitis by perforation of a hollow organ was made. From a biological point of view, the blood count showed white blood cells at 24,000/mm3 with neutrophil predominance, hemoglobin level was at 16 g/dl and platelets at 520,000/mm3. A biological inflammatory syndrome was found with a C-reactive protein (CRP) of 60 mg/l. The patient was transferred to the pediatric surgery department of Charles de Gaulle Pediatric Teaching Hospital.
Therapeutic intervention: the surgical operation was carried out at three days of life. Under general anesthesia and tracheal intubation, the laparotomy by transverse incision under the umbilical allowed the exit of fecaloid liquid aspirated to approximately 10 cc. Exploration revealed a gastric perforation, oval about 1.5 cm long axis, located on the greater curvature in the middle part of the anterior face with contours of an inflammatory aspect (Figure 2). Traces of fecal matter were found scattered in the abdomen. The procedures performed were excision of the edges of the perforation followed by suture with 4/0 resorbable thread, change of the nasogastric tube, cleansing of the peritoneal cavity with warm isotonic saline (SSI). A Delhet blade was placed for drainage, then closure of the abdominal wall in three planes, followed by the dressing. No incident or accident was noted intraoperatively. A minimum fast of 04 days has been recommended.
Follow-up and outcomes: after 24 hours in the recovery room without returning to consciousness, the patient was transferred to intensive care, where he remained in a state of coma until his death on the sixth day of life and on three days postoperatively.
Patient consent: the newborns father gave his consent for the use of the data for scientific purposes.
The frequency of spontaneous gastric perforation is estimated at 1 in 2900 births, it represents 10 to 15% of gastrointestinal perforations in newborns and children [1,2]. Since the description of the first case by Siebold in 1926, several sightings have been reported around the world [1,2,4,7,8]. In black Africa few cases have been published to our knowledge, Nafatalewa in the Democratic Republic of Congo in 2017 reported one case and Coulibaly in Mali in 2019 reported two cases [5,6]. We report the 4th case in a male newborn on the first day of life. Yang, who has published one of the largest series in the literature, 68 cases collected over 36 years, report an average age of 4 days with extremes of 1 and 30 days [3]. Although neonatal gastric perforation is the prerogative of newborns, iatrogenic gastric perforation secondary to instrument use can occur at any age, particularly in young infants [2]. Like our observation, we note a recurrence of the male sex in the cases described. A sex ratio of 3 has been reported in rare series [3,4]. Although they are distinguished in spontaneous and traumatic perforation, the etiology seems multifactorial. Particularly high gastric acidity during the first week of life, in a still fragile stomach, is a contributing factor, as are abnormalities of the gastric muscle [1-4]. Underlying pathologies have been reported, these are oeso-tracheal fistula, in particular type III where mechanical ventilation can lead to gastric over-distension followed by perforation due to oeso-tracheal communication [7]. Gastric perforation is often associated with congenital gastrointestinal pathologies in approximately 21% of cases, the most common of which is intestinal malrotation [3]. In the vast majority of cases, as in our patient, no abnormality was found. However, the non-availability of anatomo-pathological examination of the edges of resection does not allow us to eliminate a potential anomaly of the gastric muscle. Prematurity seems to be a factor of vulnerability, as well as low birth weight. In premature babies, it would have a mortality 4.21 times higher than in full-term newborns [1,3,4]. Our patient was born at term, 38 weeks of amenorrhea with a good weight, 2800 g. The absence of obvious predisposing factors in our case does not allow us to formally rule out traumatic perforation, especially since the revealing abdominal distension sometimes occurred after the placement of the nasogastric tube. The clinical manifestations found are those of a common peritonitis with abdominal distension, an occlusive syndrome, respiratory distress, an infectious syndrome, diarrhea and vomiting as well as a state of shock [1,3,7].
The main differential diagnosis is necrotizing enterocolitis [2]. However, the main symptom in the newborn is abdominal distension, and it is often the chronology of its occurrence in relation to any instrumentation that often brings to mind the traumatic cause [2,7,8]. Besides this argument, in the case of Sinnathamby, it is the size and location of the perforation, close to those of the feeding tube, which led to the suspicion of an iatrogenic perforation [2]. It is never certain and remains a suspicion. If the so-called spontaneous perforation disarms the caregiver; Iatrogenic perforation could be avoided by respecting simple rules for measuring the parameters of the newborn and by adapting the dimensions of the tubes as well as the type of tube, particularly in premature babies [2,7]. This is what Sinnathamby recalls in his writing "watch your digits to avoid gastric perforation of the newborn by the feeding tube" [2]. Plain radiography of the abdomen is the key diagnostic examination, showing more or less abundant pneumoperitoneum [1,4,7,8]. In the case of massive pneumoperitoneum as in our case the upright position, vertical horizontal director ray makes it possible to see a gaseous crescent under uni or bilateral phrenic [6,9]. In the event of scanty gas effusion, the lateral decubitus lateral X-ray, horizontal ray allows the diagnosis to be made [1,7]. In some cases the pneumoperitoneum may be missing and if the clinic is strongly in favor of peritonitis, surgical management should not be delayed [2]. The first successful surgery in newborns was described by Léger in 1950 [1,2]. Since then the surgical technique has evolved little, it is the resection of the edges of the perforation followed by suturing. This technique, used by most teams, was successfully implemented in our patient [1,2]. Intraoperatively, the perforation often sits on the greater curvature and the diameter of the orifice is variable, ranging from a few millimeters to several centimeters [1,3,6]. Our patient did not wake up in postoperative intensive care. In resuscitation technical platforms more extensive than ours, the prognosis for this pathology has improved considerably, going from 100% mortality in the 1980s to 16.7% today [3].
Gastric perforation in newborns is a rare but serious pathology. The causes are multifactorial. It should be suspected in neonates with sudden abdominal distension. Plain abdominal X-ray is the key to diagnosis by showing pneumoperitoneum. The prognosis depends on the speed of treatment and perioperative resuscitation.
The authors declare no competing interests.
All the authors have read and agreed to the final manuscript.
Figure 1: plain abdominal radiography realised at D1 of life showing a massive hydro pneumoperitoneum with a nasogastric tube in place: 1) massive pneumoperitoneum extend to the flanks and subphrenic regions; 2) intraperitoneal free fluid; (white arrow): nasogastric tube with its distal end in the stomach; 3) stomach located in the left flank pushed by the pneumoperitoneum
Figure 2: intraoperative picture at three days of life showing the gastric perforation (black arrow)
- Wineni LPNP, Setiawan A. Gastric perforation in a 5-day-old infant: A case report. Med J Malaysia. 2022 Jul;77(Suppl 1):28-30. PubMed | Google Scholar
- Sinnathamby A, Low JM, Dale Lincoln Ser Keng L, Yvonne Peng Mei N. Watch your numbers! Avoiding gastric perforation from feeding tubes in neonates. Pediatr Neonatol. 2021 Nov;62(6):681-2. PubMed | Google Scholar
- Yang T, Huang Y, Li J, Zhong W, Tan T, Yu J et al. Neonatal Gastric Perforation: Case Series and Literature Review. World J Surg. 2018 Aug;42(8):2668-73. PubMed | Google Scholar
- Pulzer F, Bennek J, Robel-Tillig E, Knüpfer M, Vogtmann C. Gastric perforation in a newborn. The Lancet. Feb 28, 2004;363(9410):703. PubMed | Google Scholar
- Coulibaly Y, Keita M, Issa Amadou, Farota S, Ouologem H, Maiga M et al. Neonatal Gastric Perforation. Mali Med. 2019;34(1):6. PubMed | Google Scholar
- Nafatalewa DK, Misenga JB, Musapudi EM, Yebalaya PM, Mujinga DT, Ilunga GN. Spontaneous neonatal gastric perforation: about a case. Pan Afr Med J. 2018;30:72. PubMed | Google Scholar
- Gupta A, Pande D, Kachru N, Khan A. Tracheoesophageal Fistula Complicated by Iatrogenic Gastric Perforation in a Low Birth Weight Neonate. J Nepal Health Res Counc. 2020;18(2):3. PubMed | Google Scholar
- Elzeneini W, Woodward C, Shalaby MS. Neonatal gastric perforation: when to expect and how to manage. Br J Hosp Med (Lond). 2019 Mar 2;80(3):i. PubMed | Google Scholar
- Ebenezer K, Bose A, Carl S. Neonatal gastric perforation following inadvertent connection of oxygen to the nasogastric feeding tube. Arch Dis Child Fetal Neonatal Ed. sept 2007;92(5):F407s. PubMed | Google Scholar
Search
This article authors
On Pubmed
On Google Scholar
Citation [Download]
Navigate this article
Similar articles in
Key words
Tables and figures
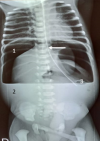 Figure 1: plain abdominal radiography realised at D1 of life showing a massive hydro pneumoperitoneum with a nasogastric tube in place: 1) massive pneumoperitoneum extend to the flanks and subphrenic regions; 2) intraperitoneal free fluid; (white arrow): nasogastric tube with its distal end in the stomach; 3) stomach located in the left flank pushed by the pneumoperitoneum
Figure 1: plain abdominal radiography realised at D1 of life showing a massive hydro pneumoperitoneum with a nasogastric tube in place: 1) massive pneumoperitoneum extend to the flanks and subphrenic regions; 2) intraperitoneal free fluid; (white arrow): nasogastric tube with its distal end in the stomach; 3) stomach located in the left flank pushed by the pneumoperitoneum