Primary brain neuroendocrine carcinoma: a case report
Samba Soumiya, Moukhlissi Mohammed, Ben Sghier Ahmed, Bouabid Meriem, Berhili Soufiane, Sami Aziz Brahmi, Mezouar Loubna
Corresponding author: Samba Soumiya, Radiotherapy Department University Mohamed VI Hospital, Faculty of Medicine and Pharmacy, Mohamed First University Oujda, Oujda, Morocco 
Received: 11 Dec 2023 - Accepted: 05 Mar 2024 - Published: 03 Apr 2024
Domain: Oncology
Keywords: Brain neuroendocrine carcinoma, radiation therapy, follow-up, case report
©Samba Soumiya et al. PAMJ Clinical Medicine (ISSN: 2707-2797). This is an Open Access article distributed under the terms of the Creative Commons Attribution International 4.0 License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Cite this article: Samba Soumiya et al. Primary brain neuroendocrine carcinoma: a case report. PAMJ Clinical Medicine. 2024;14:35. [doi: 10.11604/pamj-cm.2024.14.35.42378]
Available online at: https://www.clinical-medicine.panafrican-med-journal.com//content/article/14/35/full
Primary brain neuroendocrine carcinoma: case report
Samba Soumiya1,&, Moukhlissi Mohammed1, Ben sghier Ahmed1, Bouabid Meriem1, ![]() Berhili Soufiane1, Sami Aziz Brahmi2, Mezouar Loubna1
Berhili Soufiane1, Sami Aziz Brahmi2, Mezouar Loubna1
&Corresponding author
Primary brain neuroendocrine carcinoma (NEC) is a rare neoplasm that arises from neuroendocrine cells, a distinct group of specialized cells. In this paper, we report a primary brain neuroendocrine carcinoma in a young patient revealed by an intracranial hypertension syndrome, diagnosed at a locally advanced stage, successfully managed by external beam radiotherapy followed by four cycles of chemotherapy with a comprehensive literature review on the primary brain neuroendocrine carcinoma.
Brain neuroendocrinecarcinomais a rare neoplasm arising from cells of the endocrine and nervous systems. While they can arise in various organs, they are most prevalent in the digestive and respiratory tracts [1]. Neuroendocrine carcinomas are known to develop in all the aforementioned systems; however, very few studies imply that neuroendocrine carcinoma largely originates from the brain due to its rarity [2]. Neuroendocrine cells naturally coordinate the production and release of physiologically active chemicals in response to neurotransmitter stimulation. As a result, neuroendocrine have distinct characteristics, including the ability to secrete physiologically active amines and peptidyl hormones, allowing for their unique identification [3].
The prevalence of neuroendocrine neoplasms (NENs) has shown a notable upward trend throughout the last two decades [4]. The numerical value provided by the user is insufficient to determine the context or subject for a phenomenon is thought to be attributable to the higher rates of detection. Although the frequency of occurrence is on the rise, these tumors remain very rare, constituting about 4%-6% of all malignancies outside the cranial cavity [3,5]. When brain NECs are identified as the initial signs of illness, it's important to rule out extracranial localizations [6]. In this paper, we discuss the case of a previously healthy 22-year-old male patient who was hospitalized with symptoms of intracranial pressure believed to be caused by a primary neuroendocrine carcinoma of the brain. Despite extensive diagnostic work, no additional potential causes of primary tumors were identified.
Patient information: a 22-year-old man, a professional worker, with no significant medical history, and no family history of cancer, was consulted following gradually worsening headaches, morning vomiting, and diplopia over the past three months. Symptomatology develops in the context of general state conservation.
Clinical findings: the clinical examination found a patient conscious, and stable in hemodynamic and respiratory terms, the rest of the examination objects no anomaly including the neurological and ganglionary examination.
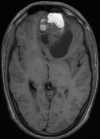
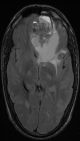
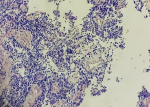
Diagnostic assessment: a magnetic resonance imaging (MRI) of the brain was performed and revealed a 7.4 cm solitary lesion in the left frontal lobe from the ethmoid region (Figure 1, Figure 2). Then a biopsy of the area between the nostrils was performed. The tissue proliferation consisted of small cells with a high nucleo-cytoplasmic ratio. These cells had hyperchromatic nuclei with distinct nucleoli. The mitotic count was estimated at fifteen mitoses per square millimeter. Initially, the tumor was described as a neuxsqroblastoma, but a referral to a specialized pathology lab described it as more consistent with an NEN. The tissue was strongly positive for synaptophysin and chromogranin, and neurofilament staining was focally positive (Figure 3). Epithelial membrane antigen (EMA), glial fibrillar acidic protein (GFAP), neuronal nuclear antigen (NeuN), S-100, thyroid transcription factor-1 (TTF1), CDX2, and a pancreatic battery including insulin, gastrin, somatostatin, glucagon, vasoactive intestinal peptide (VIP) and pancreatic polypeptide (PP) were all negative, which favored a neuroendocrine carcinoma over neuroblastoma.
Therapeutic interventions: the patient received firstly radiation therapy on brain injury at a total dose of 54 Gy in 30 fractions, then 4 cycles of chemotherapy based on cisplatin and etoposide.
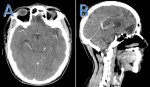
Follow-up and outcomes: radiation therapy and chemotherapy were delivered without major incidents, and monitoring during treatment showed improvement in clinical symptoms including the disappearance of headaches and improvement of diplopia, after 3 months of the end of treatment a radiological evaluation was done by a cerebral scanner (Figure 4) that showed a decrease in the size of the cerebral tumor process in favor of a favorable response; the patient is still alive and good control of his disease.
Patient perspective: the patient was satisfied with the quality of care and therapeutic outcomes.
Informed consent: the patient was informed about the procedure of publishing a research paper and gave consent.
Primary intracranial neuroendocrine carcinomas are extremely rare, and only a few cases have been reported in the literature. No deaths have been attributed to this condition. Most cases are situated in the skull base and sellar regions, with isolated instances in the third ventricle, frontal convexity, parietal lobe, and cerebellum [7]. Neuroendocrine carcinoma can be clinically categorized as functional or non-functional based on hormone production. They can also be categorized by anatomical location and grade. Ki-67, expressed in actively dividing cells, indicates the degree of proliferation. Proper classification of neuroendocrine carcinoma aids in determining diagnostic methods, treatment options, and disease prognosis [8]. The comprehensive evaluation of neuroendocrine neoplasms often involves the use of computed tomography scans and fluorodeoxyglucose positron emissiontomography/computed tomography (FDG PET/CT) scans. The sensitivity of computed tomography (CT) imaging in the detection of a primary tumor in cases with unknown primary neuroendocrine neoplasms (NEN) is reported to be 95% [9].
Our patient hadundergoing brain magnetic resonance imaging (MRI) and whole-body computed tomography (CT) [10]. Based on the pathological characteristics observed in this case, we agree that the differential diagnosis of neuroendocrine tumors (NECs) should encompass esthesioneuroblastoma and ectopic sparsely granulated corticotroph adenoma (SGCA). In our case at first, the medical diagnosis indicated the presence of an esthesio-neuroblastoma, however; further examination of the tissue revealed strong positive results for synaptophysin and chromogranin, with focal positivity for neurofilament staining. These findings suggested the presence of a neuroendocrine carcinoma. Additional tests, including EMA, GFAP, NeuN, S-100, TTF1, CDX2, and a pancreatic battery consisting of insulin, gastrin, somatostatin, glucagon, VIP, and PP, all yielded negative results. This helped to eliminate the possibility of several other primary brain neoplasms and extracranial neuroendocrine neoplasms [3]. Intracranial involvement by neuroendocrine carcinoma is commonly seen as hematogenous metastasis.
The average time between initial diagnosis and presentation of intracranial involvement is around 13 months. Brain metastases in individuals with NECs are less than 5%. Notably, only about 1.4% of metastatic brain tumors are classified as NECs, with the majority originating from the lung. Gender doesn't significantly influence the incidence of brain metastases. Patients with metastatic neuroendocrine neoplasms in the brain often have additional local and distant metastases [1]. Further investigation is required about the appropriate management of primary brain neuroendocrine neoplasms. Based on existing evidence, it seems that radiation therapy and surgery may provide benefits in the treatment of brain neuroendocrine carcinomas. Moreover, it is recommended to use platinum-based chemotherapy as a treatment option for high-grade neuroendocrine carcinomas. In this instance, the patient under consideration had radiation treatment, followed by a combination of cisplatin and etoposide, resulting in beneficial outcomes. Therefore, this technique may be regarded as a potential therapy option for primary neuroendocrine carcinomas of the brain [10]. The prognosis of individuals with brain neuroendocrine neoplasms might significantly vary from that of metastatic brain neuroendocrine carcinomass due to the primary cause of death being attributed to systemic disease progression. Primary brain neuroendocrine carcinoma has a greater resemblance to non-metastatic neuroendocrine carcinoma, whereby the 10-year overall survival rate is at 47% [3].
Primary brain neuroendocrine are infrequent pathological abnormalities. The first therapeutic approach for this particular kind of tumor involves surgical intervention, which is afterward complemented by radiation therapy and chemotherapy. The prognosis of malignant malignancies is associated with their histological grade. The instance that was described was a high-grade malignancy with a restricted outlook.
The authors declare no competing interests.
All the authors have read and agreed to the final manuscript.
Figure 1: brain magnetic resonance imaging (MRI) cuts axial t1 shows a frontal process with a double hypointense carnal and liquid hypo signal component
Figure 2: cerebral magnetic resonance imaging (MRI) cuts axial t2 flair that shows a frontal tumor process with the double component hypointense and high signal liquid cranium
Figure 3: histological image of a neuroendocrine brain tumor
Figure 4: CT scan of brain neuroendocrine carcinoma that showed a decrease in the size of the cerebral tumor process: A) transversal section; B) sagittal section
- Malenka RC, Nestler EJ, Hyman SE, Sydor A, Brown RY. Molecular neuropharmacology: a foundation for clinical neuroscience. NY: McGraw-Hill Medical. 2009. Google Scholar
- Porter DG, Chakrabarty A, McEvoy A, Bradford R. Intracranial carcinoid without evidence of extracranial disease. Neuropathol Appl Neurobiol. 2000 Jun;26(3):298-300. PubMed | Google Scholar
- Hallet J, Law CH, Cukier M, Saskin R, Liu N, Singh S. Exploring the rising incidence of neuroendocrine tumors: A population-based analysis of epidemiology, metastatic presentation, and outcomes. Cancer. 2015 Feb 15;121(4):589-97. PubMed | Google Scholar
- Dasari A, Shen C, Halperin D, Zhao B, Zhou S, Xu Y et al. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncology. 2017;3(10):1335-1342. PubMed | Google Scholar
- Hauso O, Gustafsson BI, Kidd M, Waldum HL, Drozdov I, Chan AKC et al. Neuroendocrine tumor epidemiology: contrasting Norway and North America. Cancer. 2008 Nov 15;113(10):2655-64. PubMed | Google Scholar
- Hlatky R, Suki D, Sawaya R. Carcinoid metastasis to the brain. Cancer. 2004 Dec 1;101(11):2605-133. PubMed | Google Scholar
- Cheng A, Barron J, Holmes O, Bartlett P, Jenkins G, Seal M. Primary neuroendocrine tumor of the pineal gland: a case report. BMC Neurol. 2021 Aug 20;21(1):323. PubMed | Google Scholar
- DeLellis RA. The neuroendocrine system and its tumors: An overview. Am J Clin Pathol. 2001 Jun;115 Suppl:S5-16. PubMed | Google Scholar
- Kirshbom PM, Kherani AR, Onaitis MW, Feldman JM, Tyler DS. Carcinoids of Unknown Origin: Comparative Analysis with Foregut, Midgut, and Hindgut Carcinoids. Surgery. 1998 Dec;124(6):1063-70. PubMed | Google Scholar
- Maxwell JE, Howe JR. Imaging in neuroendocrine tumors: an update for the clinician. Int J Endocr Oncol. 2015;2(02):159-168. PubMed | Google Scholar